当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Role of Delocalization Energy on Superhalogen Property: The Electron Affinity of , , and (X=O, S, and Se)
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-04-01 , DOI: 10.1002/slct.202000449 Hossein Farrokhpour 1 , Mostafa Yousefvand 1 , Hassan Hadadzadeh 1 , Hamidreza Jouypazadeh 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-04-01 , DOI: 10.1002/slct.202000449 Hossein Farrokhpour 1 , Mostafa Yousefvand 1 , Hassan Hadadzadeh 1 , Hamidreza Jouypazadeh 1
Affiliation
|
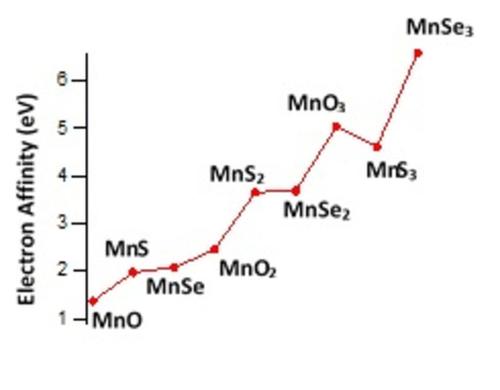
The electron affinities (EAs) of manganese compounds including ,
,  and
and  (X=O, S, and Se; n=0 to 6) species were calculated. The calculated EAs of neutral species are positive, while the EA values of anions remain negative. The increase of the EA value among the number of X atoms for the neutral species was observed so that
(X=O, S, and Se; n=0 to 6) species were calculated. The calculated EAs of neutral species are positive, while the EA values of anions remain negative. The increase of the EA value among the number of X atoms for the neutral species was observed so that  (EA=+5.04 eV),
(EA=+5.04 eV),  (EA=+4.61 eV), and
(EA=+4.61 eV), and  (EA=+6.58 eV) all revealed superhalogen property. To interpret the variation of EA with n and number of X atoms, the change in the partial charges of atoms, the electrostatic surface potential (ESP) of species, the strength of Mn−X bonds, the delocalization energy, and the energy components of electronic energy of species were examined. The positive EA of some neutral species is attributed to the increase of delocalization energy due to the added electron. In contrast, for some species, the decrease of delocalization energy is observed despite having a positive EA. For these species, the decomposition of electronic energies provides a reasonable interpretation of the positive EA value.
(EA=+6.58 eV) all revealed superhalogen property. To interpret the variation of EA with n and number of X atoms, the change in the partial charges of atoms, the electrostatic surface potential (ESP) of species, the strength of Mn−X bonds, the delocalization energy, and the energy components of electronic energy of species were examined. The positive EA of some neutral species is attributed to the increase of delocalization energy due to the added electron. In contrast, for some species, the decrease of delocalization energy is observed despite having a positive EA. For these species, the decomposition of electronic energies provides a reasonable interpretation of the positive EA value.
中文翻译:

离域能量对超卤素性质的作用:,和(X = O,S和Se)的电子亲和力
计算了包括 ,和(X = O,S和Se; n = 0到6)物种的锰化合物的电子亲和力(EA s)。所计算的EA中性物质为正,而EA阴离子的值保持为负。观察到中性物种的X原子数目中EA值增加,因此(EA = + 5.04 eV),(EA = + 4.61 eV)和(EA = + 6.58 eV)均显示出超卤素性质。用n解释EA的变化




 检查X原子的数量和数量,原子的部分电荷的变化,物质的静电表面电势(ESP),Mn-X键的强度,离域能和物质的电子能级。一些中性物种的正EA归因于由于添加电子而引起的离域能的增加。相反,对于某些物种,尽管EA为正,但仍观察到离域能量的降低。对于这些物种,电子能量的分解为正EA值提供了合理的解释。
检查X原子的数量和数量,原子的部分电荷的变化,物质的静电表面电势(ESP),Mn-X键的强度,离域能和物质的电子能级。一些中性物种的正EA归因于由于添加电子而引起的离域能的增加。相反,对于某些物种,尽管EA为正,但仍观察到离域能量的降低。对于这些物种,电子能量的分解为正EA值提供了合理的解释。
更新日期:2020-04-01
 ,
,  and
and  (X=O, S, and Se; n=0 to 6) species were calculated. The calculated EAs of neutral species are positive, while the EA values of anions remain negative. The increase of the EA value among the number of X atoms for the neutral species was observed so that
(X=O, S, and Se; n=0 to 6) species were calculated. The calculated EAs of neutral species are positive, while the EA values of anions remain negative. The increase of the EA value among the number of X atoms for the neutral species was observed so that  (EA=+5.04 eV),
(EA=+5.04 eV),  (EA=+4.61 eV), and
(EA=+4.61 eV), and  (EA=+6.58 eV) all revealed superhalogen property. To interpret the variation of EA with n and number of X atoms, the change in the partial charges of atoms, the electrostatic surface potential (ESP) of species, the strength of Mn−X bonds, the delocalization energy, and the energy components of electronic energy of species were examined. The positive EA of some neutral species is attributed to the increase of delocalization energy due to the added electron. In contrast, for some species, the decrease of delocalization energy is observed despite having a positive EA. For these species, the decomposition of electronic energies provides a reasonable interpretation of the positive EA value.
(EA=+6.58 eV) all revealed superhalogen property. To interpret the variation of EA with n and number of X atoms, the change in the partial charges of atoms, the electrostatic surface potential (ESP) of species, the strength of Mn−X bonds, the delocalization energy, and the energy components of electronic energy of species were examined. The positive EA of some neutral species is attributed to the increase of delocalization energy due to the added electron. In contrast, for some species, the decrease of delocalization energy is observed despite having a positive EA. For these species, the decomposition of electronic energies provides a reasonable interpretation of the positive EA value.
中文翻译:

离域能量对超卤素性质的作用:,和(X = O,S和Se)的电子亲和力
计算了包括 ,和(X = O,S和Se; n = 0到6)物种的锰化合物的电子亲和力(EA s)。所计算的EA中性物质为正,而EA阴离子的值保持为负。观察到中性物种的X原子数目中EA值增加,因此(EA = + 5.04 eV),(EA = + 4.61 eV)和(EA = + 6.58 eV)均显示出超卤素性质。用n解释EA的变化





 检查X原子的数量和数量,原子的部分电荷的变化,物质的静电表面电势(ESP),Mn-X键的强度,离域能和物质的电子能级。一些中性物种的正EA归因于由于添加电子而引起的离域能的增加。相反,对于某些物种,尽管EA为正,但仍观察到离域能量的降低。对于这些物种,电子能量的分解为正EA值提供了合理的解释。
检查X原子的数量和数量,原子的部分电荷的变化,物质的静电表面电势(ESP),Mn-X键的强度,离域能和物质的电子能级。一些中性物种的正EA归因于由于添加电子而引起的离域能的增加。相反,对于某些物种,尽管EA为正,但仍观察到离域能量的降低。对于这些物种,电子能量的分解为正EA值提供了合理的解释。











































 京公网安备 11010802027423号
京公网安备 11010802027423号