当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Triazole‐fused indolo[2,3‐a]carbazoles: synthesis, structures, and properties
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-03-31 , DOI: 10.1002/jhet.3974 Sirina Ghosh 1 , Sarasija Das 1 , Chandan Kumar 1 , Neha Rani Kumar 1 , Abhijeet R. Agrawal 1 , Himadri S. Karmakar 1 , Nani Gopal Ghosh 1 , Sanjio S. Zade 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-03-31 , DOI: 10.1002/jhet.3974 Sirina Ghosh 1 , Sarasija Das 1 , Chandan Kumar 1 , Neha Rani Kumar 1 , Abhijeet R. Agrawal 1 , Himadri S. Karmakar 1 , Nani Gopal Ghosh 1 , Sanjio S. Zade 1
Affiliation
|
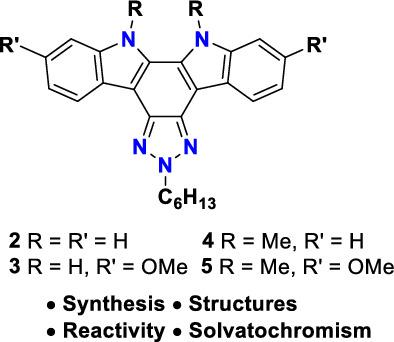
The synthesis, structure, and photophysical and electrochemical properties of triazole fused indolo[2,3‐a ]carbazole derivatives 2 ‐5 are reported. The key step involved in the synthesis of triazole fused indolo[2,3‐a ]carbazole derivatives is the Cadogan ring closing reaction. 2‐Hexyl‐5,6‐dinitro‐2H ‐benzo[d ][1,2,3]triazoles having 4,7‐diaryl capping were subjected to the Cadogan cyclization reaction to obtain compounds 2‐5 . In contrast to thiadiazole‐fused indolo[2,3‐a ]carbazole 1 , bromination of triazole‐fused indolo[2,3‐a ]carbazole 4 afforded only meta‐dibrominated product with respect to the nitrogen of fused pyrrole rings on treatment with both N ‐bromosuccinimide (NBS) and elemental bromine. These compounds showed positive solvatochromism in their emission spectra. Incorporation of electron‐donating substituent in the indole moiety resulted in the elevation of the highest occupied molecular orbital (HOMO) level. Density functional theory (DFT) calculations were performed to support the experimental findings.
中文翻译:

三唑稠合的吲哚[2,3-a]咔唑:合成,结构和性质
的合成,结构,和光物理和电化学三唑的特性吲哚并稠合[2,3-一个]咔唑衍生物2 - 5中报告。合成三唑稠合的吲哚[2,3- a ]咔唑衍生物的关键步骤是Cadogan闭环反应。将具有4,7-二芳基封端的2-己基-5,6-二硝基-2 H-苯并[ d ] [1,2,3]三唑进行Cadogan环化反应,以获得化合物2-5。与噻二唑稠合的吲哚[2,3‐ a ]咔唑1相比,三唑稠合的吲哚[2,3‐ a ]咔唑4的溴化在使用N-溴代琥珀酰亚胺(NBS)和元素溴处理时,仅提供了与稠合吡咯环的氮有关的间二溴化产物。这些化合物在其发射光谱中显示出正溶剂溶变色。吲哚部分中给电子取代基的引入导致最高占据分子轨道(HOMO)的水平升高。进行密度泛函理论(DFT)计算以支持实验结果。
更新日期:2020-03-31
中文翻译:

三唑稠合的吲哚[2,3-a]咔唑:合成,结构和性质
的合成,结构,和光物理和电化学三唑的特性吲哚并稠合[2,3-一个]咔唑衍生物2 - 5中报告。合成三唑稠合的吲哚[2,3- a ]咔唑衍生物的关键步骤是Cadogan闭环反应。将具有4,7-二芳基封端的2-己基-5,6-二硝基-2 H-苯并[ d ] [1,2,3]三唑进行Cadogan环化反应,以获得化合物2-5。与噻二唑稠合的吲哚[2,3‐ a ]咔唑1相比,三唑稠合的吲哚[2,3‐ a ]咔唑4的溴化在使用N-溴代琥珀酰亚胺(NBS)和元素溴处理时,仅提供了与稠合吡咯环的氮有关的间二溴化产物。这些化合物在其发射光谱中显示出正溶剂溶变色。吲哚部分中给电子取代基的引入导致最高占据分子轨道(HOMO)的水平升高。进行密度泛函理论(DFT)计算以支持实验结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号