当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational investigation of the ligand effect on the chemo/regioselectivity and reactivity of cobalt-catalysed hydroformylation
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-04-01 , DOI: 10.1039/c9cy02562f Pan Li 1, 2, 3, 4, 5 , Chaoren Shen 5, 6, 7, 8, 9 , Jie Min 1, 2, 3, 4, 5 , Jing-Yuan Mei 1, 2, 3, 4, 5 , Huan Zheng 5, 6, 7, 8, 9 , Lin He 5, 6, 7, 8, 9 , Xinxin Tian 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-04-01 , DOI: 10.1039/c9cy02562f Pan Li 1, 2, 3, 4, 5 , Chaoren Shen 5, 6, 7, 8, 9 , Jie Min 1, 2, 3, 4, 5 , Jing-Yuan Mei 1, 2, 3, 4, 5 , Huan Zheng 5, 6, 7, 8, 9 , Lin He 5, 6, 7, 8, 9 , Xinxin Tian 1, 2, 3, 4, 5
Affiliation
|
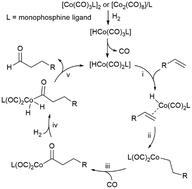
The ligand effect on the chemo/regioselectivity and reactivity of cobalt-catalysed hydroformylation has been discussed. The results of the unmodified cobalt carbonyl catalyst showed that the regioselectivity of the terminal alkene substrate was mainly affected by the steric hindrance of the bulky alkyl substitution group and was insensitive to the elongation of the carbon chain. Regarding the addition of Co–H onto the alkene, modifying the cobalt carbonyl catalyst with a phosphine ligand led to more distinct differences between the Markovnikov and anti-Markovnikov pathways with respect to both the energy barrier and the reaction free energy. Among the four selected phosphines, PBu3 and a5-PhobPC5 with large Tolman cone angles favoured anti-Markovnikov-type Co–H addition, which is beneficial for the generation of a linear product. The modification with phosphine also promoted the oxidative addition of H2 on the Co(I) centre by lowering the energy barrier of H2 splitting and generating a more stable Co(III)-dihydride complex but retarded the reductive elimination step by elevating its activation energy and reaction energy. On the whole, the hydroformylation mechanism is similar for both modified and unmodified cobalt carbonyl catalysts. Moreover, PBu3 modification on the catalyst does not intrinsically change the chemoselectivity of alkene but indeed improves the subsequent alcohol and formic ester formation.
中文翻译:

配体对钴催化加氢甲酰化反应的化学/区域选择性和反应性的影响的计算研究
讨论了配体对钴催化加氢甲酰化反应的化学/区域选择性和反应性的影响。未改性的羰基钴催化剂的结果表明,末端烯烃底物的区域选择性主要受庞大的烷基取代基的空间位阻的影响,并且对碳链的延伸不敏感。关于将Co-H加到烯烃上,用膦配体改性羰基钴催化剂导致Markovnikov和反Markovnikov途径在能垒和反应自由能方面存在更明显的差异。在四种选定的膦中,PBu 3和5- PhobPC 5Tolman锥角较大的情况更有利于添加反Markovnikov型Co-H,这对于线性产物的生成是有利的。用膦进行的修饰还通过降低H 2分解的能垒并生成更稳定的Co(III)-二氢化物配合物来促进H 2在Co(I)中心的氧化加成反应,但通过提高其活化性而延迟了还原消除步骤能量和反应能量。总体而言,改性和未改性羰基钴催化剂的加氢甲酰化机理均相似。此外,PBu 3 催化剂上的改性并不能本质上改变烯烃的化学选择性,但实际上可以改善随后的醇和甲酸酯的形成。
更新日期:2020-04-01
中文翻译:

配体对钴催化加氢甲酰化反应的化学/区域选择性和反应性的影响的计算研究
讨论了配体对钴催化加氢甲酰化反应的化学/区域选择性和反应性的影响。未改性的羰基钴催化剂的结果表明,末端烯烃底物的区域选择性主要受庞大的烷基取代基的空间位阻的影响,并且对碳链的延伸不敏感。关于将Co-H加到烯烃上,用膦配体改性羰基钴催化剂导致Markovnikov和反Markovnikov途径在能垒和反应自由能方面存在更明显的差异。在四种选定的膦中,PBu 3和5- PhobPC 5Tolman锥角较大的情况更有利于添加反Markovnikov型Co-H,这对于线性产物的生成是有利的。用膦进行的修饰还通过降低H 2分解的能垒并生成更稳定的Co(III)-二氢化物配合物来促进H 2在Co(I)中心的氧化加成反应,但通过提高其活化性而延迟了还原消除步骤能量和反应能量。总体而言,改性和未改性羰基钴催化剂的加氢甲酰化机理均相似。此外,PBu 3 催化剂上的改性并不能本质上改变烯烃的化学选择性,但实际上可以改善随后的醇和甲酸酯的形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号