Nature ( IF 50.5 ) Pub Date : 2020-04-01 , DOI: 10.1038/s41586-020-2131-1 Ian A MacKenzie 1 , Leifeng Wang 1 , Nicholas P R Onuska 1 , Olivia F Williams 1 , Khadiza Begam 2 , Andrew M Moran 1 , Barry D Dunietz 3 , David A Nicewicz 1
|
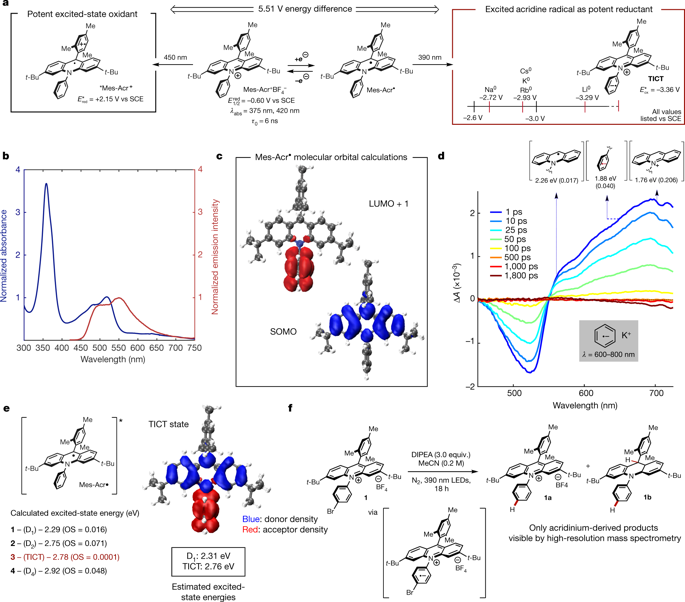
Photoinduced electron transfer (PET) is a phenomenon whereby the absorption of light by a chemical species provides an energetic driving force for an electron-transfer reaction1,2,3,4. This mechanism is relevant in many areas of chemistry, including the study of natural and artificial photosynthesis, photovoltaics and photosensitive materials. In recent years, research in the area of photoredox catalysis has enabled the use of PET for the catalytic generation of both neutral and charged organic free-radical species. These technologies have enabled previously inaccessible chemical transformations and have been widely used in both academic and industrial settings. Such reactions are often catalysed by visible-light-absorbing organic molecules or transition-metal complexes of ruthenium, iridium, chromium or copper5,6. Although various closed-shell organic molecules have been shown to behave as competent electron-transfer catalysts in photoredox reactions, there are only limited reports of PET reactions involving neutral organic radicals as excited-state donors or acceptors. This is unsurprising because the lifetimes of doublet excited states of neutral organic radicals are typically several orders of magnitude shorter than the singlet lifetimes of known transition-metal photoredox catalysts7,8,9,10,11. Here we document the discovery, characterization and reactivity of a neutral acridine radical with a maximum excited-state oxidation potential of −3.36 volts versus a saturated calomel electrode, which is similarly reducing to elemental lithium, making this radical one of the most potent chemical reductants reported12. Spectroscopic, computational and chemical studies indicate that the formation of a twisted intramolecular charge-transfer species enables the population of higher-energy doublet excited states, leading to the observed potent photoreducing behaviour. We demonstrate that this catalytically generated PET catalyst facilitates several chemical reactions that typically require alkali metal reductants and can be used in other organic transformations that require dissolving metal reductants.
中文翻译:

吖啶自由基光还原剂的发现和表征
光致电子转移 (PET) 是一种现象,化学物质对光的吸收为电子转移反应提供能量驱动力1,2,3,4. 这种机制与许多化学领域相关,包括自然和人工光合作用、光伏和光敏材料的研究。近年来,光氧化还原催化领域的研究使 PET 能够用于催化生成中性和带电的有机自由基物种。这些技术使以前无法实现的化学转化成为可能,并已广泛用于学术和工业环境。这种反应通常由吸收可见光的有机分子或钌、铱、铬或铜的过渡金属配合物催化5,6. 尽管各种闭壳有机分子已被证明在光氧化还原反应中充当有效的电子转移催化剂,但涉及中性有机自由基作为激发态供体或受体的 PET 反应的报道有限。这并不奇怪,因为中性有机自由基的双重激发态的寿命通常比已知的过渡金属光氧化还原催化剂的单重激发态寿命短几个数量级7,8,9,10,11。在这里,我们记录了最大激发态氧化电位为 -3.36 伏的中性吖啶自由基与饱和甘汞电极的发现、表征和反应性,后者同样还原为元素锂,使该自由基成为最有效的化学还原剂之一报道12 . 光谱、计算和化学研究表明,扭曲的分子内电荷转移物质的形成能够产生更高能量的双重激发态,从而导致观察到有效的光还原行为。我们证明了这种催化生成的 PET 催化剂促进了几种通常需要碱金属还原剂的化学反应,并可用于其他需要溶解金属还原剂的有机转化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号