JAMA Dermatology ( IF 11.5 ) Pub Date : 2020-05-01 , DOI: 10.1001/jamadermatol.2020.0035 Megan Arthur 1 , Nicole M Fett 2 , Emile Latour 3 , Heidi Jacobe 4 , Elaine Kunzler 4 , Stephanie Florez-Pollack 4 , Jacob Houser 4 , Shivani Sharma 4 , Smriti Prasad 4 , Alisa Femia 5 , Marleigh J Stern 5 , Lisa K Pappas-Taffer 6 , Rebecca Gaffney 6 , Anthony P Fernandez 7 , Daniel Knabel 7 , Adela Rambi Cardones 8 , Nicole Leung 8 , Anne Laumann 9 , Jeong Min Yu 9 , Jeffrey Zhao 9 , Ruth Ann Vleugels 10 , Elizabeth Tkachenko 10 , Kelly Lo 10
|
Importance First-line systemic therapy for morphea includes methotrexate with or without systemic corticosteroids. When this regimen is ineffective, not tolerated, or contraindicated, a trial of mycophenolate mofetil (MMF) or mycophenolic acid (MPA)—referred to herein as mycophenolate—is recommended; however, evidence to support this recommendation remains weak.
Objective To evaluate the effectiveness and tolerability of mycophenolate for the treatment of morphea.
Design, Setting, and Participants A retrospective cohort study was conducted from January 1, 1999, to December 31, 2018, among 77 patients with morphea from 8 institutions who were treated with mycophenolate.
Main Outcomes and Measures The primary outcome was morphea disease activity, severity, and response at 0, 3 to 6, and 9 to 12 months of mycophenolate treatment. A secondary outcome was whether mycophenolate was a well-tolerated treatment of morphea.
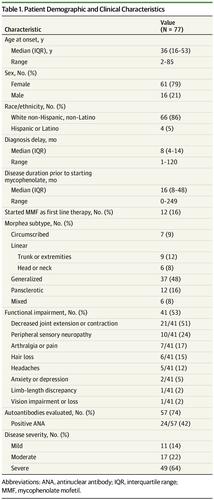
Results There were 61 female patients (79%) and 16 male patients (21%) in the study, with a median age at disease onset of 36 years (interquartile range, 16-53 years) and median diagnostic delay of 8 months (interquartile range, 4-14 months). Generalized morphea (37 [48%]), pansclerotic morphea (12 [16%]), and linear morphea of the trunk and/or extremities (9 [12%]) were the most common subtypes of morphea identified. Forty-one patients (53%) had an associated functional impairment, and 49 patients (64%) had severe disease. Twelve patients received initial treatment with mycophenolate as monotherapy or combination therapy and 65 patients received mycophenolate after prior treatment was ineffective (50 of 65 [77%]) or poorly tolerated (21 of 65 [32%]). Treatments prior to mycophenolate included methotrexate (48 of 65 [74%]), systemic corticosteroids (42 of 65 [65%]), hydroxychloroquine (20 of 65 [31%]), and/or phototherapy (14 of 65 [22%]). After 3 to 6 months of mycophenolate treatment, 66 of 73 patients had stable (n = 22) or improved (n = 44) disease. After 9 to 12 months of treatment, 47 of 54 patients had stable (n = 14) or improved (n = 33) disease. Twenty-seven patients (35%) achieved disease remission at completion of the study. Treatments received in conjunction with mycophenolate were frequent. Mycophenolate was well tolerated. Gastrointestinal adverse effects were the most common (24 [31%]); cytopenia (3 [4%]) and infection (2 [3%]) occurred less frequently.
Conclusions and Relevance This study suggests that mycophenolate is a well-tolerated and beneficial treatment of recalcitrant, severe morphea.
中文翻译:

霉酚酸酯和霉酚酸治疗硬斑病的有效性和耐受性评价。
重要性 硬斑病的一线全身治疗包括甲氨蝶呤联合或不联合全身性皮质类固醇。当该方案无效、不能耐受或存在禁忌症时,建议试用吗替麦考酚酯 (MMF) 或霉酚酸 (MPA)——本文称为霉酚酸酯;然而,支持这一建议的证据仍然薄弱。
目的 评价霉酚酸酯治疗硬斑病的有效性和耐受性。
设计、地点和参与者 一项回顾性队列研究于 1999 年 1 月 1 日至 2018 年 12 月 31 日期间进行,研究对象为来自 8 个机构的 77 名接受霉酚酸酯治疗的硬斑病患者。
主要结果和测量 主要结果是霉菌病活动度、严重程度以及霉酚酸酯治疗第 0、3 至 6 个月和 9 至 12 个月时的反应。次要结果是霉酚酸酯是否是一种耐受性良好的硬斑病治疗药物。
结果 研究中有 61 名女性患者 (79%) 和 16 名男性患者 (21%),中位发病年龄为 36 岁(四分位距,16-53 岁),中位诊断延迟时间为 8 个月(四分位距) , 4-14 个月)。全身性硬斑病 (37 [48%])、泛硬化性硬斑病 (12 [16%]) 和躯干和/或四肢线状硬斑病 (9 [12%]) 是最常见的硬斑病亚型。41 名患者 (53%) 有相关的功能障碍,49 名患者 (64%) 有严重疾病。12 名患者接受霉酚酸酯作为单一疗法或联合疗法的初始治疗,65 名患者在先前治疗无效(65 名中的 50 名 [77%])或耐受性差(65 名中的 21 名 [32%])后接受霉酚酸酯。霉酚酸酯之前的治疗包括甲氨蝶呤(65 例中的 48 例 [74%])、全身性皮质类固醇(65 人中的 42 人 [65%])、羟氯喹(65 人中的 20 人 [31%])和/或光疗(65 人中的 14 人 [22%])。在霉酚酸酯治疗 3 至 6 个月后,73 名患者中有 66 名病情稳定 (n = 22) 或好转 (n = 44)。经过 9 到 12 个月的治疗,54 名患者中有 47 名病情稳定 (n = 14) 或好转 (n = 33)。27 名患者 (35%) 在研究完成时实现了疾病缓解。与霉酚酸酯一起接受的治疗很常见。霉酚酸酯耐受性良好。胃肠道不良反应最常见 (24 [31%]);血细胞减少 (3 [4%]) 和感染 (2 [3%]) 的发生频率较低。经过 9 到 12 个月的治疗,54 名患者中有 47 名病情稳定 (n = 14) 或好转 (n = 33)。27 名患者 (35%) 在研究完成时实现了疾病缓解。与霉酚酸酯一起接受的治疗很常见。霉酚酸酯耐受性良好。胃肠道不良反应最常见 (24 [31%]);血细胞减少 (3 [4%]) 和感染 (2 [3%]) 的发生频率较低。经过 9 到 12 个月的治疗,54 名患者中有 47 名病情稳定 (n = 14) 或好转 (n = 33)。27 名患者 (35%) 在研究完成时实现了疾病缓解。与霉酚酸酯一起接受的治疗很常见。霉酚酸酯耐受性良好。胃肠道不良反应最常见 (24 [31%]);血细胞减少 (3 [4%]) 和感染 (2 [3%]) 的发生频率较低。
结论和相关性 本研究表明霉酚酸酯是一种耐受性良好且有益的治疗顽固性严重硬斑病的药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号