JAMA Surgery ( IF 15.7 ) Pub Date : 2020-06-01 , DOI: 10.1001/jamasurg.2019.6361 Julie A Margenthaler 1
|
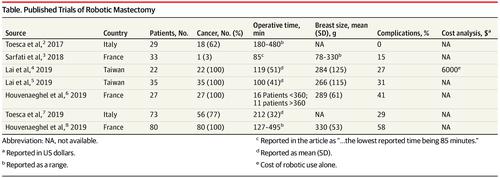
Robotic-assisted nipple-sparing mastectomy was first reported in a cadaveric study conducted by Sarfati et al1 in 2016. Four fresh cadaveric breasts were used, and the authors reported that the procedure was feasible with complete mammary gland removal through a 4-cm axillary line incision.1 Soon after that initial report, other groups from Europe and Asia reported similar investigations in which patients were undergoing nipple-sparing mastectomy either for breast cancer prevention (in carriers of gene variations) or for treatment of newly diagnosed breast cancer.2-4 Currently, the use of the robot to assist in mastectomy procedures is not approved by the US Food and Drug Administration. Despite this, there have been isolated reports of robotic-assisted breast surgery in the US in the past 2 years. In response to these reports, the US Food and Drug Administration issued a statement on February 28, 2019, warning that the safety and effectiveness of robotic devices for mastectomy have not been established.
中文翻译:

机器人乳房切除术程序故障?
Sarfati等[ 1]在2016年进行的尸体研究中首次报道了机器人辅助的保留乳头乳房切除术。使用了四个新鲜的尸体乳房,作者报告说该程序可行,可通过4厘米腋窝完全切除乳腺线切口。1这份初步报告发表后不久,来自欧洲和亚洲的其他小组也进行了类似的研究,其中患者正在接受保留乳头的乳房切除术,以预防乳腺癌(在基因变异的携带者中)或治疗新诊断的乳腺癌。2 -4目前,美国食品药品监督管理局尚未批准使用机器人协助乳房切除术。尽管如此,在过去的2年中,美国仍没有关于机器人辅助乳房手术的报道。针对这些报告,美国食品药品监督管理局于2019年2月28日发表声明,警告尚未确定用于乳房切除术的机器人设备的安全性和有效性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号