Molecular Therapy: Oncology ( IF 5.3 ) Pub Date : 2020-03-31 , DOI: 10.1016/j.omto.2020.03.016 Takeshi Koujima 1 , Hiroshi Tazawa 1, 2 , Takeshi Ieda 1 , Hiroyuki Araki 1 , Takuro Fushimi 1 , Ryohei Shoji 1 , Shinji Kuroda 1, 2 , Satoru Kikuchi 1, 3 , Ryuichi Yoshida 1 , Yuzo Umeda 1 , Fuminori Teraishi 1 , Yasuo Urata 4 , Hiroyuki Mizuguchi 5 , Toshiyoshi Fujiwara 1
|
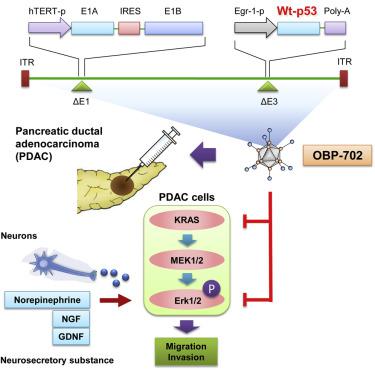
Pancreatic ductal adenocarcinoma (PDAC) cells have an exceptional ability to invade nerves through pronounced crosstalk between nerves and cancer cells; however, the mechanism of PDAC cell invasion remains to be elucidated. Here, we demonstrate the therapeutic potential of telomerase-specific oncolytic adenoviruses, OBP-301 and tumor suppressor p53-armed OBP-702, against human PDAC cells. Highly invasive PDAC cells exhibited higher levels of phosphorylated extracellular signal-regulated kinases 1 and 2 (ERK1/2) expression independent of KRAS expression; ERK1/2 inhibitor or small interfering RNA (siRNA) treatment significantly reduced the migration and invasion of PDAC cells, suggesting that the ERK signaling pathway is associated with the invasiveness of PDAC cells. OBP-702 infection suppressed ERK signaling and inhibited PDAC cell migration and invasion more efficiently than OBP-301. OBP-702 also effectively inhibited PDAC cell invasion even when invasiveness was enhanced by administration of motility stimulators, such as nerve and neurosecretory factors. Moreover, noninvasive whole-body imaging analyses showed that OBP-702 significantly suppressed tumor growth in an orthotopic PDAC xenograft model, although both viruses were equally effective against subcutaneous tumors, suggesting that OBP-702 can influence the orthotopic tumor microenvironment. Our data suggest that oncolytic virus-mediated disruption of ERK signaling is a promising antitumor strategy for attenuating the invasiveness of PDAC cells.
中文翻译:

溶瘤病毒介导的ERK信号通路靶向抑制人类胰腺癌的侵袭倾向。
胰腺导管腺癌(PDAC)细胞具有通过神经与癌细胞之间明显的串扰侵入神经的出色能力。但是,PDAC细胞入侵的机制仍有待阐明。在这里,我们证明了端粒酶特异性溶瘤腺病毒OBP-301和抑癌p53武装的OBP-702对人PDAC细胞的治疗潜力。高侵害性的PDAC细胞表现出较高水平的磷酸化细胞外信号调节激酶1和2(ERK1 / 2)表达,而与KRAS表达无关。ERK1 / 2抑制剂或小干扰RNA(siRNA)处理可显着减少PDAC细胞的迁移和侵袭,提示ERK信号通路与PDAC细胞的侵袭性有关。与OBP-301相比,OBP-702感染可更有效地抑制ERK信号传导并抑制PDAC细胞迁移和侵袭。即使通过施用诸如神经和神经分泌因子之类的运动刺激剂来增强侵袭性,OBP-702也可以有效抑制PDAC细胞侵袭。此外,无创全身成像分析显示,OBP-702在原位PDAC异种移植模型中显着抑制了肿瘤的生长,尽管两种病毒对皮下肿瘤均具有同等效力,这表明OBP-702可以影响原位肿瘤的微环境。我们的数据表明溶瘤病毒介导的ERK信号转导是一种有希望的抗肿瘤策略,可减轻PDAC细胞的侵袭性。即使通过施用诸如神经和神经分泌因子之类的运动刺激剂来增强侵袭性,OBP-702也可以有效抑制PDAC细胞侵袭。此外,非侵入性全身成像分析显示,OBP-702在原位PDAC异种移植模型中显着抑制了肿瘤的生长,尽管两种病毒对皮下肿瘤均具有同等效力,这表明OBP-702可以影响原位肿瘤的微环境。我们的数据表明溶瘤病毒介导的ERK信号转导是一种有希望的抗肿瘤策略,可减轻PDAC细胞的侵袭性。即使通过施用诸如神经和神经分泌因子之类的运动刺激剂来增强侵袭性,OBP-702也可以有效抑制PDAC细胞侵袭。此外,非侵入性全身成像分析显示,OBP-702在原位PDAC异种移植模型中显着抑制了肿瘤的生长,尽管两种病毒对皮下肿瘤均具有同等效力,这表明OBP-702可以影响原位肿瘤的微环境。我们的数据表明溶瘤病毒介导的ERK信号转导是一种有希望的抗肿瘤策略,可减轻PDAC细胞的侵袭性。非侵入性全身成像分析显示,尽管两种病毒对皮下肿瘤均具有同等效力,但是OBP-702在原位PDAC异种移植模型中显着抑制了肿瘤的生长,这表明OBP-702可以影响原位肿瘤的微环境。我们的数据表明溶瘤病毒介导的ERK信号转导是一种有希望的抗肿瘤策略,可减轻PDAC细胞的侵袭性。非侵入性全身成像分析显示,虽然两种病毒对皮下肿瘤均具有同等效力,但OBP-702在原位PDAC异种移植模型中可显着抑制肿瘤生长,这表明OBP-702可以影响原位肿瘤微环境。我们的数据表明溶瘤病毒介导的ERK信号转导是一种有希望的抗肿瘤策略,可减轻PDAC细胞的侵袭性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号