当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluation of cell surface reactive immuno-adjuvant in combination with immunogenic cell death inducing drug for in situ chemo-immunotherapy.
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-03-31 , DOI: 10.1016/j.jconrel.2020.03.029 Adam A Walters 1 , Julie Tzu-Wen Wang 1 , Khuloud T Al-Jamal 1
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-03-31 , DOI: 10.1016/j.jconrel.2020.03.029 Adam A Walters 1 , Julie Tzu-Wen Wang 1 , Khuloud T Al-Jamal 1
Affiliation
|
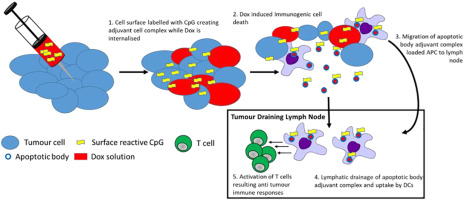
Apoptotic cells and cell fragments, especially those produced as a result of immunogenic cell death (ICD), are known to be a potential source of cancer vaccine immunogen. However, due to variation between tumours and between individuals, methods to generate such preparations may require extensive ex vivo personalisation. To address this, we have utilised the concept of in situ vaccination whereby an ICD inducing drug is injected locally to generate immunogenic apoptotic fragments/cells. These fragments are then adjuvanted by a co-administered cell reactive CpG adjuvant. We first evaluate means of labelling tumour cells with CpG adjuvant, we then go on to demonstrate in vitro that labelling is preserved following apoptosis and, furthermore, that the apoptotic body-adjuvant complexes are readily transferred to macrophages. In in vivo studies we observe synergistic tumour growth delays and elevated levels of CD4+ and CD8+ cells in tumours receiving adjuvant drug combination. CD4+/CD8+ cells are likewise elevated in the tumour draining lymph node and activated to a greater extent than individual treatments. This study represents the first steps toward the evaluation of rationally formulated drug-adjuvant combinations for in situ chemo-immunotherapy.
中文翻译:

细胞表面反应性免疫佐剂与免疫原性细胞死亡诱导药物联合用于原位化学免疫治疗的评估。
已知凋亡细胞和细胞碎片,尤其是由于免疫原性细胞死亡(ICD)产生的细胞凋亡和细胞碎片,是癌症疫苗免疫原的潜在来源。然而,由于肿瘤之间和个体之间的差异,产生这种制剂的方法可能需要广泛的离体个性化。为了解决这个问题,我们利用了原位疫苗接种的概念,其中将ICD诱导药物局部注射以产生免疫原性凋亡片段/细胞。然后通过共同施用的细胞反应性CpG佐剂佐剂这些片段。我们首先评估用CpG佐剂标记肿瘤细胞的方法,然后我们继续在体外证明细胞凋亡后标记被保留,此外,凋亡的身体佐剂复合物很容易转移到巨噬细胞。在体内研究中,我们观察到在接受辅助药物联合治疗的肿瘤中肿瘤的协同生长延迟和CD4 +和CD8 +细胞水平升高。CD4 + / CD8 +细胞同样在引流肿瘤的淋巴结中升高,并且其活化程度高于单独治疗。这项研究代表了评估原位化学免疫疗法合理配制的药物-佐剂组合的第一步。
更新日期:2020-04-01
中文翻译:

细胞表面反应性免疫佐剂与免疫原性细胞死亡诱导药物联合用于原位化学免疫治疗的评估。
已知凋亡细胞和细胞碎片,尤其是由于免疫原性细胞死亡(ICD)产生的细胞凋亡和细胞碎片,是癌症疫苗免疫原的潜在来源。然而,由于肿瘤之间和个体之间的差异,产生这种制剂的方法可能需要广泛的离体个性化。为了解决这个问题,我们利用了原位疫苗接种的概念,其中将ICD诱导药物局部注射以产生免疫原性凋亡片段/细胞。然后通过共同施用的细胞反应性CpG佐剂佐剂这些片段。我们首先评估用CpG佐剂标记肿瘤细胞的方法,然后我们继续在体外证明细胞凋亡后标记被保留,此外,凋亡的身体佐剂复合物很容易转移到巨噬细胞。在体内研究中,我们观察到在接受辅助药物联合治疗的肿瘤中肿瘤的协同生长延迟和CD4 +和CD8 +细胞水平升高。CD4 + / CD8 +细胞同样在引流肿瘤的淋巴结中升高,并且其活化程度高于单独治疗。这项研究代表了评估原位化学免疫疗法合理配制的药物-佐剂组合的第一步。











































 京公网安备 11010802027423号
京公网安备 11010802027423号