当前位置:
X-MOL 学术
›
J. Colloid Interface Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Band bending of TiO2 induced by O-xylene and acetaldehyde adsorption and its effect on the generation of active radicals.
Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2020-03-31 , DOI: 10.1016/j.jcis.2020.03.114 Qinglong Zeng 1 , Xiao Wang 2 , Xiaofeng Xie 2 , Asad Mahmood 2 , Guanhong Lu 2 , Yan Wang 2 , Jing Sun 2
Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2020-03-31 , DOI: 10.1016/j.jcis.2020.03.114 Qinglong Zeng 1 , Xiao Wang 2 , Xiaofeng Xie 2 , Asad Mahmood 2 , Guanhong Lu 2 , Yan Wang 2 , Jing Sun 2
Affiliation
|
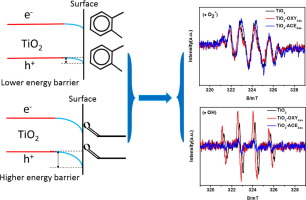
Most studies on the photodegradation of volatile organic compounds (VOCs) have focused on the synthesis of efficient photocatalysts. However, little attention has been paid to the band bending change of semiconductive photocatalysts after the adsorption of VOCs. Herein, we first disclose how the adsorption of two typical VOCs influences the band bending of P-type rutile TiO2 and consequently changes the amount of reactive radicals. This provides a new way to understand the experimental phenomenon of heterogeneous reactions. Theoretical computations of the adsorption model and zeta potential tests both verified that o-xylene is an acceptor molecule when it adsorbs on the TiO2 surface, and it tends to attract electrons from TiO2. In contrast, acetaldehyde is a donor molecule. A distinct electron transfer direction between TiO2 and adsorbed molecules (o-xylene and acetaldehyde) induces a different band bending degree. O-xylene adsorption alleviates the downward band bending of TiO2 itself, whereas acetaldehyde adsorption strengthens the downward band bending. The probability of electrons and holes reaching the TiO2 surface is influenced by this change, which has a considerable influence on the generation of active radicals. Consequently, o-xylene adsorption leads to more hydroxyl radical generation, and acetaldehyde adsorption results in less hydroxyl radical generation. As a result, hydroxyl radicals play the predominant role in the degradation of o-xylene, whereas the photocatalysis of acetaldehyde is dominant for superoxide radicals. In addition, the band bending of a semiconductor induced by gaseous molecule adsorption has the potential for application in gas sensors to improve sensitivity.
中文翻译:

邻二甲苯和乙醛吸附引起的TiO2的能带弯曲及其对活性自由基产生的影响。
关于挥发性有机化合物(VOC)的光降解的大多数研究都集中在合成有效的光催化剂上。然而,很少有人关注吸附VOCs后半导体光催化剂的能带弯曲变化。在这里,我们首先公开两种典型VOC的吸附如何影响P型金红石型TiO2的能带弯曲,从而改变反应性自由基的数量。这提供了一种理解异质反应实验现象的新方法。吸附模型的理论计算和ζ电势测试均证实,邻二甲苯在吸附到TiO2表面时是一个受体分子,并且倾向于从TiO2吸引电子。相反,乙醛是供体分子。TiO2与吸附的分子(邻二甲苯和乙醛)之间明显的电子转移方向引起不同的能带弯曲度。邻二甲苯吸附可减轻TiO2本身的向下弯曲,而乙醛吸附则可增强向下弯曲。电子和空穴到达TiO2表面的可能性受此变化的影响,这对活性自由基的产生有很大影响。因此,邻二甲苯吸附导致更多的羟基自由基生成,乙醛吸附导致较少的羟基自由基生成。结果,羟基自由基在邻二甲苯的降解中起主要作用,而乙醛的光催化作用对于超氧自由基是主要的。此外,
更新日期:2020-04-01
中文翻译:

邻二甲苯和乙醛吸附引起的TiO2的能带弯曲及其对活性自由基产生的影响。
关于挥发性有机化合物(VOC)的光降解的大多数研究都集中在合成有效的光催化剂上。然而,很少有人关注吸附VOCs后半导体光催化剂的能带弯曲变化。在这里,我们首先公开两种典型VOC的吸附如何影响P型金红石型TiO2的能带弯曲,从而改变反应性自由基的数量。这提供了一种理解异质反应实验现象的新方法。吸附模型的理论计算和ζ电势测试均证实,邻二甲苯在吸附到TiO2表面时是一个受体分子,并且倾向于从TiO2吸引电子。相反,乙醛是供体分子。TiO2与吸附的分子(邻二甲苯和乙醛)之间明显的电子转移方向引起不同的能带弯曲度。邻二甲苯吸附可减轻TiO2本身的向下弯曲,而乙醛吸附则可增强向下弯曲。电子和空穴到达TiO2表面的可能性受此变化的影响,这对活性自由基的产生有很大影响。因此,邻二甲苯吸附导致更多的羟基自由基生成,乙醛吸附导致较少的羟基自由基生成。结果,羟基自由基在邻二甲苯的降解中起主要作用,而乙醛的光催化作用对于超氧自由基是主要的。此外,











































 京公网安备 11010802027423号
京公网安备 11010802027423号