当前位置:
X-MOL 学术
›
J. Colloid Interface Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring the gel phase of cationic glycylalanylglycine in ethanol/water. II. Spectroscopic, kinetic and thermodynamic studies.
Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2020-03-31 , DOI: 10.1016/j.jcis.2020.03.108 David M DiGuiseppi 1 , Lavenia Thursch 2 , Nicolas J Alvarez 2 , Reinhard Schweitzer-Stenner 1
Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2020-03-31 , DOI: 10.1016/j.jcis.2020.03.108 David M DiGuiseppi 1 , Lavenia Thursch 2 , Nicolas J Alvarez 2 , Reinhard Schweitzer-Stenner 1
Affiliation
|
HYPOTHESIS
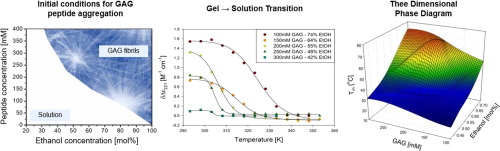
Recently, we reported a three-dimensional phase diagram for the gelation of cationic tripeptide glycylalanylglycine (GAG) in water-ethanol mixtures. We showed that the gel strength reaches an optimum for a peptide concentration of 200 mM and ethanol/water mixtures with ca. 55-60 mol% ethanol. An increase of the ethanol fraction causes a substantial upshift of the gel's softening temperature which is indicative of a reduced peptide solubility. We expect the formation of long crystalline fibrils which form the sample spanning network of the gel phase to precede the gelation process and that the fibril microstructure depends on the rate and concentration of peptide.
EXPERIMENTS
We used UV circular dichroism (UVCD) spectroscopy to probe the kinetics of GAG fibril formation as a function of peptide concentration and ethanol fraction. We provide experimental evidence for the notion that the utilized CD signal reflects the three-dimensional assembly of peptides rather than a two-dimensional sheet structure. UVCD was also used to probe the melting of GAG fibrils with increasing temperature. FTIR and vibrational circular dichroism (VCD) spectroscopy were employed to characterize the structure of sheets with which the observed fibrils were formed.
FINDINGS
Fibrilization and gelation kinetics occur on a very similar time scale for very short gelation times (<7 min) observed at high peptide concentrations and/or ethanol fractions. Otherwise, gelation proceeds significantly slower than fibrilization. The trends in the UVCD spectral response parallel the trends in the storage modulus as a function of peptide concentration and ethanol fraction. IR and VCD profiles of amide I' reveal that fibril structure and the respective chirality are both affected by peptide concentration and solvent composition. At high ethanol fractions, the VCD changes its sign suggesting a conversion from phase II to phase I. Generally, the latter is obtained only at temperatures below 15 °C. Altogether, our results reveal how GAG fibrilization and gelation are interrelated and how the gel properties can be tuned by changing the composition of the ternary GAG/water/ethanol mixture.
中文翻译:

探索在乙醇/水中的阳离子甘氨酰丙氨酰甘氨酸的凝胶相。二。光谱,动力学和热力学研究。
假设最近,我们报道了在水-乙醇混合物中阳离子三肽甘氨酰丙氨酰甘氨酸(GAG)凝胶化的三维相图。我们表明,对于200 mM的肽浓度和大约20的乙醇/水混合物,凝胶强度达到最佳。55-60mol%的乙醇。乙醇分数的增加会导致凝胶的软化温度大幅上升,这表明肽的溶解度降低。我们期望形成长的结晶原纤维,该原纤维形成样品跨过凝胶相的网络,从而先于凝胶化过程,并且原纤维的微观结构取决于肽的速率和浓度。实验我们使用紫外圆二色性(UVCD)光谱来探测GAG原纤维形成动力学与肽浓度和乙醇分数的关系。我们为利用的CD信号反映了肽的三维组装而不是二维的片状结构这一概念提供了实验证据。UVCD还被用来探测温度升高时GAG原纤维的熔化。FTIR和振动圆二色性(VCD)光谱用于表征形成观察到的原纤维的片的结构。结果在高肽浓度和/或乙醇馏分下观察到的非常短的胶凝时间(<7分钟)中,纤维化和胶凝动力学发生的时间非常相似。否则,凝胶化的进行明显比原纤维化慢。UVCD光谱响应的趋势与储能模量的趋势(与肽浓度和乙醇分数的函数)平行。酰胺I'的IR和VCD轮廓 结果表明原纤维结构和各自的手性均受肽浓度和溶剂组成的影响。在乙醇含量高的情况下,VCD会改变其符号,表明从II相转变为I相。通常,仅在15°C以下的温度才能获得后者。总而言之,我们的结果揭示了GAG的纤维化和凝胶化是如何相互关联的,以及如何通过改变三元GAG /水/乙醇混合物的组成来调节凝胶特性。
更新日期:2020-04-01
中文翻译:

探索在乙醇/水中的阳离子甘氨酰丙氨酰甘氨酸的凝胶相。二。光谱,动力学和热力学研究。
假设最近,我们报道了在水-乙醇混合物中阳离子三肽甘氨酰丙氨酰甘氨酸(GAG)凝胶化的三维相图。我们表明,对于200 mM的肽浓度和大约20的乙醇/水混合物,凝胶强度达到最佳。55-60mol%的乙醇。乙醇分数的增加会导致凝胶的软化温度大幅上升,这表明肽的溶解度降低。我们期望形成长的结晶原纤维,该原纤维形成样品跨过凝胶相的网络,从而先于凝胶化过程,并且原纤维的微观结构取决于肽的速率和浓度。实验我们使用紫外圆二色性(UVCD)光谱来探测GAG原纤维形成动力学与肽浓度和乙醇分数的关系。我们为利用的CD信号反映了肽的三维组装而不是二维的片状结构这一概念提供了实验证据。UVCD还被用来探测温度升高时GAG原纤维的熔化。FTIR和振动圆二色性(VCD)光谱用于表征形成观察到的原纤维的片的结构。结果在高肽浓度和/或乙醇馏分下观察到的非常短的胶凝时间(<7分钟)中,纤维化和胶凝动力学发生的时间非常相似。否则,凝胶化的进行明显比原纤维化慢。UVCD光谱响应的趋势与储能模量的趋势(与肽浓度和乙醇分数的函数)平行。酰胺I'的IR和VCD轮廓 结果表明原纤维结构和各自的手性均受肽浓度和溶剂组成的影响。在乙醇含量高的情况下,VCD会改变其符号,表明从II相转变为I相。通常,仅在15°C以下的温度才能获得后者。总而言之,我们的结果揭示了GAG的纤维化和凝胶化是如何相互关联的,以及如何通过改变三元GAG /水/乙醇混合物的组成来调节凝胶特性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号