Gastroenterology ( IF 25.7 ) Pub Date : 2020-04-01 , DOI: 10.1053/j.gastro.2020.03.053 Siyuan Ding 1 , Yanhua Song 2 , Kevin F Brulois 3 , Junliang Pan 3 , Julia Y Co 4 , Lili Ren 5 , Ningguo Feng 6 , Linda L Yasukawa 6 , Liliana Sánchez-Tacuba 6 , Jonathan E Wosen 7 , Elizabeth D Mellins 7 , Denise M Monack 8 , Manuel R Amieva 4 , Calvin J Kuo 9 , Eugene C Butcher 3 , Harry B Greenberg 6
|
Background & Aims
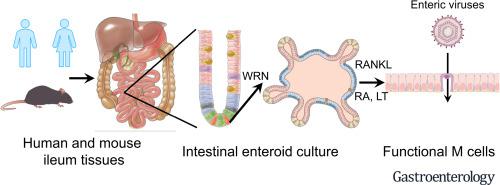
Intestinal microfold (M) cells are a unique subset of intestinal epithelial cells in the Peyer’s patches that regulate mucosal immunity, serving as portals for sampling and uptake of luminal antigens. The inability to efficiently develop human M cells in cell culture has impeded studies of the intestinal immune system. We aimed to identify signaling pathways required for differentiation of human M cells and establish a robust culture system using human ileum enteroids.
Methods
We analyzed transcriptome data from mouse Peyer’s patches to identify cell populations in close proximity to M cells. We used the human enteroid system to determine which cytokines were required to induce M-cell differentiation. We performed transcriptome, immunofluorescence, scanning electron microscope, and transcytosis experiments to validate the development of phenotypic and functional human M cells.
Results
A combination of retinoic acid and lymphotoxin induced differentiation of glycoprotein 2-positive human M cells, which lack apical microvilli structure. Upregulated expression of innate immune-related genes within M cells correlated with a lack of viral antigens after rotavirus infection. Human M cells, developed in the enteroid system, internalized and transported enteric viruses, such as rotavirus and reovirus, across the intestinal epithelium barrier in the enteroids.
Conclusions
We identified signaling pathways required for differentiation of intestinal M cells, and used this information to create a robust culture method to develop human M cells with capacity for internalization and transport of viruses. Studies of this model might increase our understanding of antigen presentation and the systemic entry of enteric pathogens in the human intestine.
中文翻译:

视黄酸和淋巴毒素信号转导促进人肠道 M 细胞的分化。
背景与目标
肠微皱襞 (M) 细胞是 Peyer 淋巴集结中肠上皮细胞的一个独特亚群,可调节粘膜免疫,作为取样和摄取管腔抗原的入口。在细胞培养中无法有效地开发人类 M 细胞阻碍了对肠道免疫系统的研究。我们旨在确定人类 M 细胞分化所需的信号通路,并使用人类回肠肠建立稳健的培养系统。
方法
我们分析了来自小鼠 Peyer 淋巴集结的转录组数据,以识别与 M 细胞非常接近的细胞群。我们使用人类肠系统来确定诱导 M 细胞分化所需的细胞因子。我们进行了转录组、免疫荧光、扫描电子显微镜和转胞吞作用实验,以验证表型和功能性人类 M 细胞的发育。
结果
视黄酸和淋巴毒素的组合诱导糖蛋白 2 阳性人 M 细胞分化,这些细胞缺乏顶端微绒毛结构。M 细胞内先天免疫相关基因的上调表达与轮状病毒感染后病毒抗原的缺乏相关。在肠样系统中发育的人 M 细胞内化并运输肠道病毒,如轮状病毒和呼肠孤病毒,穿过肠样中的肠上皮屏障。
结论
我们确定了肠道 M 细胞分化所需的信号通路,并利用该信息创建了一种稳健的培养方法,以开发具有病毒内化和运输能力的人类 M 细胞。对该模型的研究可能会增加我们对抗原呈递和肠道病原体进入人体肠道的了解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号