当前位置:
X-MOL 学术
›
Free Radical Bio. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The uptake mechanism and intracellular fate of Paraoxonase-1 in endothelial cells.
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-03-31 , DOI: 10.1016/j.freeradbiomed.2020.03.032 Ben-David Raz 1 , Chuyun Dimitry 2 , Szuchman-Sapir Andrea 1
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-03-31 , DOI: 10.1016/j.freeradbiomed.2020.03.032 Ben-David Raz 1 , Chuyun Dimitry 2 , Szuchman-Sapir Andrea 1
Affiliation
|
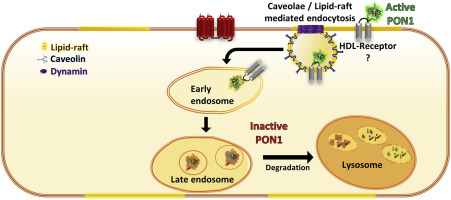
Paraoxonase-1 (PON1) is a high-density lipoprotein (HDL)-associated lactonase that plays a significant role in the anti-atherosclerotic activity of HDL. However, several studies have shown that PON1 localizes in cells, where it operates independently of HDL. Previously, we showed that PON1 localizes in endothelial cells (ECs), and impairs vasodilation mediated by the endothelium-derived hyperpolarizing factor (EDHF) 5,6-δ-DHTL. However, the internalization pathway of PON1 into ECs, and the intracellular fate of PON1 are unknown. Therefore, the present study aimed to elucidate the uptake mechanism, intracellular trafficking and the function of PON1 in ECs. We conducted a series of inhibition experiments of fluorescently labeled recombinant PON1 (rePON1) in ECs, followed by FACS analyses. We found that rePON1 binds the EC membrane via specific binding sites located in lipid-rafts/caveolae microdomains that are shared with HDL, and internalized through dynamin-dependent endocytosis. Qualitative assessments of the intracellular trafficking of rePON1, using confocal z-stack images, showed colocalization of the labeled rePON1 with early and late endosome/lysosome markers. Accordingly, a "pulse-chase" incubation of rePON1, followed by lactonase activity measurement in EC lysate, revealed that rePON1 retains its lactonase activity after binding to the cells. However, this activity decreases over time. Finally, induction of endothelial dysfunction with high glucose, angiotensin II, or palmitic acid increased rePON1 uptake by ECs. In conclusion, these results indicate that free PON1 interacts with ECs via binding sites located in lipid-rafts/caveolae, where it is enzymatically active and regulates endothelial functions. However, once internalized, PON1 is degraded. Additionally, alteration in endothelial function affects PON1 uptake by ECs.
中文翻译:

对氧磷酶-1在内皮细胞中的摄取机制和细胞内命运。
对氧磷酶-1(PON1)是高密度脂蛋白(HDL)相关的内酯酶,在HDL的抗动脉粥样硬化活性中起重要作用。但是,一些研究表明PON1定位于细胞中,而该细胞独立于HDL运行。以前,我们表明PON1定位于内皮细胞(EC),并损害由内皮衍生的超极化因子(EDHF)5,6-δ-DHTL介导的血管舒张。但是,PON1进入EC的内在途径以及PON1的细胞内命运尚不清楚。因此,本研究旨在阐明内皮细胞的摄取机制,细胞内运输和PON1的功能。我们进行了一系列在EC中荧光标记的重组PON1(rePON1)的抑制实验,然后进行了FACS分析。我们发现rePON1通过位于HDL共享的脂筏/腔微域中的特定结合位点结合EC膜,并通过依赖于动力的内吞作用而被内化。使用共聚焦z堆栈图像对rePON1的细胞内运输进行定性评估,显示标记的rePON1与早期和晚期内体/溶酶体标记物共定位。因此,rePON1的“脉冲追踪”孵育,然后在EC裂解物中测量内酯酶活性,发现rePON1与细胞结合后保留了其内酯酶活性。但是,此活动会随着时间而减少。最后,高糖,血管紧张素II或棕榈酸对内皮功能障碍的诱导会增加ECs对REPON1的吸收。结论,这些结果表明,游离的PON1通过位于脂筏/小泡中的结合位点与EC相互作用,在那儿它具有酶促活性并调节内皮功能。但是,一旦内部化,PON1就会降级。此外,内皮功能的改变会影响EC摄取PON1。
更新日期:2020-04-01
中文翻译:

对氧磷酶-1在内皮细胞中的摄取机制和细胞内命运。
对氧磷酶-1(PON1)是高密度脂蛋白(HDL)相关的内酯酶,在HDL的抗动脉粥样硬化活性中起重要作用。但是,一些研究表明PON1定位于细胞中,而该细胞独立于HDL运行。以前,我们表明PON1定位于内皮细胞(EC),并损害由内皮衍生的超极化因子(EDHF)5,6-δ-DHTL介导的血管舒张。但是,PON1进入EC的内在途径以及PON1的细胞内命运尚不清楚。因此,本研究旨在阐明内皮细胞的摄取机制,细胞内运输和PON1的功能。我们进行了一系列在EC中荧光标记的重组PON1(rePON1)的抑制实验,然后进行了FACS分析。我们发现rePON1通过位于HDL共享的脂筏/腔微域中的特定结合位点结合EC膜,并通过依赖于动力的内吞作用而被内化。使用共聚焦z堆栈图像对rePON1的细胞内运输进行定性评估,显示标记的rePON1与早期和晚期内体/溶酶体标记物共定位。因此,rePON1的“脉冲追踪”孵育,然后在EC裂解物中测量内酯酶活性,发现rePON1与细胞结合后保留了其内酯酶活性。但是,此活动会随着时间而减少。最后,高糖,血管紧张素II或棕榈酸对内皮功能障碍的诱导会增加ECs对REPON1的吸收。结论,这些结果表明,游离的PON1通过位于脂筏/小泡中的结合位点与EC相互作用,在那儿它具有酶促活性并调节内皮功能。但是,一旦内部化,PON1就会降级。此外,内皮功能的改变会影响EC摄取PON1。











































 京公网安备 11010802027423号
京公网安备 11010802027423号