当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rationally Designed Small-Molecule Inhibitors Targeting an Unconventional Pocket on the TLR8 Protein-Protein Interface.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-04-13 , DOI: 10.1021/acs.jmedchem.9b02128 Shuangshuang Jiang , Hiromi Tanji 1 , Kejun Yin , Shuting Zhang , Kentaro Sakaniwa 1 , Jian Huang , Yi Yang , Jing Li 2 , Umeharu Ohto 1 , Toshiyuki Shimizu 1 , Hang Yin
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-04-13 , DOI: 10.1021/acs.jmedchem.9b02128 Shuangshuang Jiang , Hiromi Tanji 1 , Kejun Yin , Shuting Zhang , Kentaro Sakaniwa 1 , Jian Huang , Yi Yang , Jing Li 2 , Umeharu Ohto 1 , Toshiyuki Shimizu 1 , Hang Yin
Affiliation
|
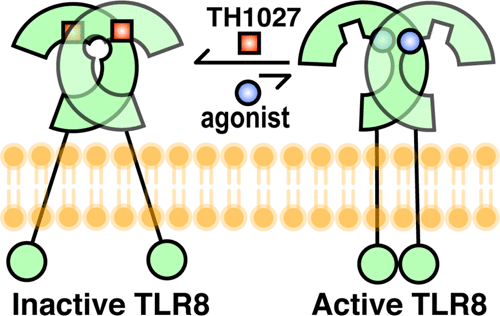
Rational designs of small-molecule inhibitors targeting protein-protein interfaces have met little success. Herein, we have designed a series of triazole derivatives with a novel scaffold to specifically intervene with the interaction of TLR8 homomerization. In multiple assays, TH1027 was identified as a highly potent and specific inhibitor of TLR8. A successful solution of the X-ray crystal structure of TLR8 in complex with TH1027 provided an in-depth mechanistic insight into its binding mode, validating that TH1027 was located between two TLR8 monomers and recognized as an unconventional pocket, thereby preventing TLR8 from activation. Further biological evaluations showed that TH1027 dose-dependently suppressed the TLR8-mediated inflammatory responses in both human monocyte cell lines, peripheral blood mononuclear cells, and rheumatoid arthritis patient specimens, suggesting a strong therapeutic potential against autoimmune diseases.
中文翻译:

合理设计的针对TLR8蛋白-蛋白质界面上非常规口袋的小分子抑制剂。
针对蛋白质-蛋白质界面的小分子抑制剂的合理设计收效甚微。在本文中,我们设计了一系列具有新型支架的三唑衍生物,以特异性地干预TLR8均化的相互作用。在多种测定中,TH1027被鉴定为TLR8的高效强特异性抑制剂。TLR8的X射线晶体结构与TH1027配合使用的成功解决方案提供了对其结合模式的深入机理研究,验证了TH1027位于两个TLR8单体之间并被认为是一个非常规的口袋,从而阻止了TLR8的活化。进一步的生物学评估表明,TH1027剂量依赖性地抑制了人类单核细胞系,外周血单核细胞和TLR8介导的炎症反应,
更新日期:2020-04-24
中文翻译:

合理设计的针对TLR8蛋白-蛋白质界面上非常规口袋的小分子抑制剂。
针对蛋白质-蛋白质界面的小分子抑制剂的合理设计收效甚微。在本文中,我们设计了一系列具有新型支架的三唑衍生物,以特异性地干预TLR8均化的相互作用。在多种测定中,TH1027被鉴定为TLR8的高效强特异性抑制剂。TLR8的X射线晶体结构与TH1027配合使用的成功解决方案提供了对其结合模式的深入机理研究,验证了TH1027位于两个TLR8单体之间并被认为是一个非常规的口袋,从而阻止了TLR8的活化。进一步的生物学评估表明,TH1027剂量依赖性地抑制了人类单核细胞系,外周血单核细胞和TLR8介导的炎症反应,











































 京公网安备 11010802027423号
京公网安备 11010802027423号