当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High-Resolution In Situ NMR Spectroscopy of Bacterial Envelope Proteins in Outer Membrane Vesicles.
Biochemistry ( IF 2.9 ) Pub Date : 2020-04-01 , DOI: 10.1021/acs.biochem.9b01123 Johannes Thoma 1, 2 , Björn M Burmann 1, 2
Biochemistry ( IF 2.9 ) Pub Date : 2020-04-01 , DOI: 10.1021/acs.biochem.9b01123 Johannes Thoma 1, 2 , Björn M Burmann 1, 2
Affiliation
|
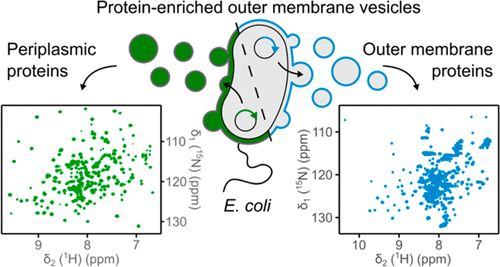
The cell envelope of Gram-negative bacteria is an elaborate cellular environment, consisting of two lipid membranes separated by the aqueous periplasm. So far, efforts to mimic this environment under laboratory conditions have been limited by the complexity of the asymmetric bacterial outer membrane. To evade this impasse, we recently established a method to modify the protein composition of bacterial outer membrane vesicles (OMVs) released from Escherichia coli as a platform for biophysical studies of outer membrane proteins in their native membrane environment. Here, we apply protein-enriched OMVs to characterize the structure of three envelope proteins from E. coli using nuclear magnetic resonance (NMR) spectroscopy and expand the methodology to soluble periplasmic proteins. We obtain high-resolution in situ NMR spectra of the transmembrane protein OmpA as well as the periplasmic proteins CpxP and MalE. We find that our approach facilitates structural investigations of membrane-attached protein domains and is especially suited for soluble proteins within their native periplasmic environment. Thereby, the use of OMVs in solution NMR methods allows in situ analysis of the structure and dynamics of proteins twice the size compared to the current in-cell NMR methodology. We therefore expect our work to pave the way for more complex NMR studies of bacterial envelope proteins in the native environment of OMVs in the future.
中文翻译:

外膜囊泡中细菌包膜蛋白的高分辨率原位核磁共振波谱分析。
革兰氏阴性细菌的细胞被膜是一个复杂的细胞环境,由两个被水性周质隔开的脂质膜组成。到目前为止,在实验室条件下模拟这种环境的努力受到不对称细菌外膜的复杂性的限制。为了避免这种僵局,我们最近建立了一种修改大肠杆菌释放的细菌外膜囊泡(OMV)蛋白质组成的方法,作为在其天然膜环境中进行外膜蛋白生物物理研究的平台。在这里,我们应用富含蛋白质的 OMV 使用核磁共振 (NMR) 光谱来表征大肠杆菌的三种包膜蛋白的结构,并将该方法扩展到可溶性周质蛋白。我们获得了跨膜蛋白 OmpA 以及周质蛋白 CpxP 和 MalE 的高分辨率原位 NMR 谱。我们发现我们的方法有助于膜附着蛋白结构域的结构研究,并且特别适合天然周质环境中的可溶性蛋白。因此,在溶液 NMR 方法中使用 OMV 可以对比当前细胞内 NMR 方法大两倍的蛋白质的结构和动力学进行原位分析。因此,我们希望我们的工作能够为未来 OMV 天然环境中细菌包膜蛋白的更复杂的 NMR 研究铺平道路。
更新日期:2020-04-01
中文翻译:

外膜囊泡中细菌包膜蛋白的高分辨率原位核磁共振波谱分析。
革兰氏阴性细菌的细胞被膜是一个复杂的细胞环境,由两个被水性周质隔开的脂质膜组成。到目前为止,在实验室条件下模拟这种环境的努力受到不对称细菌外膜的复杂性的限制。为了避免这种僵局,我们最近建立了一种修改大肠杆菌释放的细菌外膜囊泡(OMV)蛋白质组成的方法,作为在其天然膜环境中进行外膜蛋白生物物理研究的平台。在这里,我们应用富含蛋白质的 OMV 使用核磁共振 (NMR) 光谱来表征大肠杆菌的三种包膜蛋白的结构,并将该方法扩展到可溶性周质蛋白。我们获得了跨膜蛋白 OmpA 以及周质蛋白 CpxP 和 MalE 的高分辨率原位 NMR 谱。我们发现我们的方法有助于膜附着蛋白结构域的结构研究,并且特别适合天然周质环境中的可溶性蛋白。因此,在溶液 NMR 方法中使用 OMV 可以对比当前细胞内 NMR 方法大两倍的蛋白质的结构和动力学进行原位分析。因此,我们希望我们的工作能够为未来 OMV 天然环境中细菌包膜蛋白的更复杂的 NMR 研究铺平道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号