当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Small-Molecule Modulators of Toll-like Receptors.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-04-01 , DOI: 10.1021/acs.accounts.9b00631 Yibo Wang 1 , Shuting Zhang 2 , Hongyuan Li 1 , Hongshuang Wang 1 , Tianshu Zhang 1 , Mark R Hutchinson 3 , Hang Yin 2 , Xiaohui Wang 1, 4
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-04-01 , DOI: 10.1021/acs.accounts.9b00631 Yibo Wang 1 , Shuting Zhang 2 , Hongyuan Li 1 , Hongshuang Wang 1 , Tianshu Zhang 1 , Mark R Hutchinson 3 , Hang Yin 2 , Xiaohui Wang 1, 4
Affiliation
|
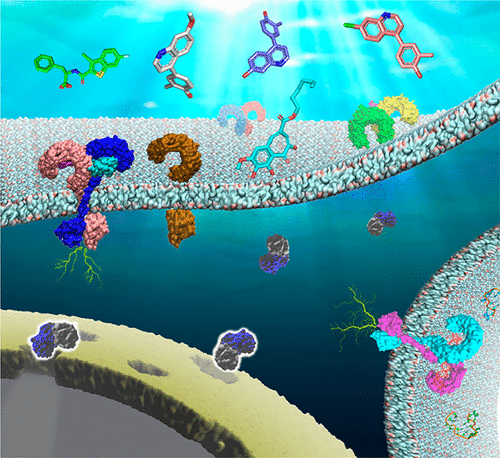
ConspectusToll-like receptors (TLRs) are the "gatekeepers" of the immune system in humans and other animals to protect the host from invading bacteria, viruses, and other microorganisms. Since TLR4 was discovered as the receptor for endotoxin in the late 1990s, significant progress has been made in exploiting an understanding of the function of TLRs. The TLR-signaling pathway is crucial for the induction and progression of various diseases. Dysregulation of TLR signaling contributes to numerous pathological conditions, including chronic inflammation, sepsis, cancers, asthma, neuropathic pain, drug addiction, and autoimmune diseases. Therefore, manipulation of TLR signaling is promising to halt their activity in inflammatory diseases, to enhance their signaling to fight cancers, to modulate their role in autoimmune diseases, and to suppress them to treat drug addiction. TLR agonists have demonstrated great potential as antimicrobial agents and vaccine adjuvants, whereas TLR antagonists are being developed as reagents and drugs to dampen immune responses. Because of their pivotal potential therapeutic applications, fruitful small-molecule compounds and peptide fragments have been discovered, and many of them have advanced to various stages of clinical trials (though only two have been approved by the Food and Drug Administration (FDA): MPLA as a TLR4 agonist and imiquimod as a TLR7 agonist).In this Account, we focus on the progress in developing TLR signaling pathway modulators (mainly focused on the Yin and Wang laboratories) over the past decade and highlight the accomplishments and currently existing challenges in the development of TLR modulators. First, we briefly describe the members of the human TLR family along with their natural modulators. Second, we illustrate our endeavors to discover TLR-targeted agents using comprehensive approaches. Specifically, a discussion of identification and characterization of new chemical entities, determination of modes of action, and further applications is presented. For instance, the TLR3 antagonist was first discovered through in silico screening, and the inhibitory activity was confirmed in murine cells. Considering the glycosylation on TLR3, a new direction for TLR3 modulator design was pointed out to target asparagine glycosylation. We have particularly focused on the discovery of TLR4 antagonists and have assessed their great potential in the clinical treatment of drug addiction and alcohol use disorders. In addition, we discuss multiple other popular and robust techniques for modulator discovery. Not only small organic modulators but also stapled peptides and peptidomimetics will attract more and more attention in the future. Finally, current challenges, opportunities, and future perspectives for TLR-targeted agents are also discussed.
中文翻译:

Toll样受体的小分子调节剂。
ConspectusToll样受体(TLR)是人类和其他动物免疫系统的“守门人”,可以保护宿主免受细菌,病毒和其他微生物的侵害。自从1990年代后期发现TLR4作为内毒素的受体以来,在对TLR功能的认识上已经取得了重大进展。TLR信号通路对于各种疾病的诱导和发展至关重要。TLR信号传导异常会导致许多病理状况,包括慢性炎症,败血症,癌症,哮喘,神经性疼痛,药物成瘾和自身免疫性疾病。因此,操纵TLR信号有望终止其在炎症性疾病中的活性,增强其与癌症作斗争的信号,调节其在自身免疫性疾病中的作用,并压制他们治疗毒品成瘾。TLR激动剂已显示出作为抗菌剂和疫苗佐剂的巨大潜力,而TLR拮抗剂正在被开发为抑制免疫反应的试剂和药物。由于其关键的潜在治疗应用,已发现了富有成果的小分子化合物和肽片段,其中许多已进入临床试验的各个阶段(尽管只有两个获得了美国食品药品管理局(FDA)的批准:MPLA在本报告中,我们重点介绍过去十年中开发TLR信号通路调节剂(主要集中在殷氏和王氏实验室)的进展,并着重介绍了成就和目前存在的挑战TLR调制器的发展。第一,我们简要描述了人类TLR家族的成员及其自然调节剂。其次,我们说明了我们使用综合方法发现以TLR为目标的代理的努力。具体而言,提出了对新化学实体的鉴定和表征,作用方式的确定以及进一步应用的讨论。例如,TLR3拮抗剂首先通过计算机筛选发现,并在鼠细胞中证实了抑制活性。考虑到TLR3上的糖基化,指出了针对TLR3调节剂设计的新方向,以靶向天冬酰胺糖基化。我们特别关注TLR4拮抗剂的发现,并评估了它们在药物成瘾和酒精使用障碍的临床治疗中的巨大潜力。此外,我们讨论了用于调制器发现的多种其他流行且健壮的技术。将来,不仅小型有机调节剂,装订的肽和拟肽也将越来越受到关注。最后,还讨论了针对TLR的代理商的当前挑战,机遇和未来前景。
更新日期:2020-04-01
中文翻译:

Toll样受体的小分子调节剂。
ConspectusToll样受体(TLR)是人类和其他动物免疫系统的“守门人”,可以保护宿主免受细菌,病毒和其他微生物的侵害。自从1990年代后期发现TLR4作为内毒素的受体以来,在对TLR功能的认识上已经取得了重大进展。TLR信号通路对于各种疾病的诱导和发展至关重要。TLR信号传导异常会导致许多病理状况,包括慢性炎症,败血症,癌症,哮喘,神经性疼痛,药物成瘾和自身免疫性疾病。因此,操纵TLR信号有望终止其在炎症性疾病中的活性,增强其与癌症作斗争的信号,调节其在自身免疫性疾病中的作用,并压制他们治疗毒品成瘾。TLR激动剂已显示出作为抗菌剂和疫苗佐剂的巨大潜力,而TLR拮抗剂正在被开发为抑制免疫反应的试剂和药物。由于其关键的潜在治疗应用,已发现了富有成果的小分子化合物和肽片段,其中许多已进入临床试验的各个阶段(尽管只有两个获得了美国食品药品管理局(FDA)的批准:MPLA在本报告中,我们重点介绍过去十年中开发TLR信号通路调节剂(主要集中在殷氏和王氏实验室)的进展,并着重介绍了成就和目前存在的挑战TLR调制器的发展。第一,我们简要描述了人类TLR家族的成员及其自然调节剂。其次,我们说明了我们使用综合方法发现以TLR为目标的代理的努力。具体而言,提出了对新化学实体的鉴定和表征,作用方式的确定以及进一步应用的讨论。例如,TLR3拮抗剂首先通过计算机筛选发现,并在鼠细胞中证实了抑制活性。考虑到TLR3上的糖基化,指出了针对TLR3调节剂设计的新方向,以靶向天冬酰胺糖基化。我们特别关注TLR4拮抗剂的发现,并评估了它们在药物成瘾和酒精使用障碍的临床治疗中的巨大潜力。此外,我们讨论了用于调制器发现的多种其他流行且健壮的技术。将来,不仅小型有机调节剂,装订的肽和拟肽也将越来越受到关注。最后,还讨论了针对TLR的代理商的当前挑战,机遇和未来前景。











































 京公网安备 11010802027423号
京公网安备 11010802027423号