当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dendrimer Conjugation Enhances Tumor Penetration and Efficacy of Doxorubicin in Extracellular Matrix-Expressing 3D Lung Cancer Models.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.molpharmaceut.0c00083 Rashed M Almuqbil , Rodrigo S Heyder , Elizabeth R Bielski , Mikhail Durymanov 1 , Joshua J Reineke 1 , Sandro R P da Rocha
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.molpharmaceut.0c00083 Rashed M Almuqbil , Rodrigo S Heyder , Elizabeth R Bielski , Mikhail Durymanov 1 , Joshua J Reineke 1 , Sandro R P da Rocha
Affiliation
|
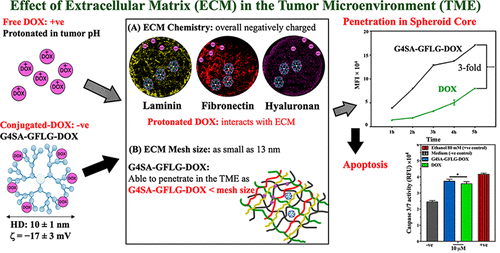
Doxorubicin (DOX) is a chemotherapeutic agent broadly used in the treatment of a range of solid tumors. In spite of its high potency, as is the case for many other chemotherapeutic drugs, there are many challenges associated with the use of DOX in clinical oncology. This is particularly true for DOX in the treatment of lung cancer, where in vitro potency is shown to be very high, but low lung distribution and off-target toxicity (particularly cardiotoxicity) restrict its use. Nanocarrier-based drug delivery systems (nanoDDS) have been shown to help alter biodistribution and alleviate off-target toxicity associated with DOX. While significant understanding exists regarding the design parameters to achieve those clinical benefits, much less is known regarding the design of nanoDDS capable of enhancing tumor penetration of DOX (and other drugs), which is another major factor leading to DOX's reduced efficacy. The purpose of this study was to design a dendrimer-based nanoDDS capable of enhancing the penetration of DOX as measured in an in vitro 3D lung tumor model and to correlate those results with its efficacy. Spheroids formed with the A549 human lung adenocarcinoma cells/murine fibroblast cell line (NIH/3T3 cell line) are shown to produce the essential components of the extracellular matrix (ECM), which is known as a physical barrier that hinders the transport of DOX. DOX was conjugated to generation 4 succinamic acid-terminated poly(amido-amine) (PAMAM) dendrimers (G4SA) through an enzyme-liable tetrapeptide (G4SA-GFLG-DOX), resulting in a nanoDDS with ∼5.5 DOX, -17 mV surface (ζ) potential, and a 10 nm hydrodynamic diameter (HD). The penetration of DOX to the core of the spheroid in terms of DOX fluorescence was determined to be 3.1-fold greater compared to free DOX, which positively correlated with enhanced efficacy as measured by the Caspase 3/7 assay. This improved penetration happens as the interactions between the G4SA-GFLG-DOX and the highly negatively charged ECM are minimized by shielding the protonatable amine of DOX upon conjugation, and the HD of the conjugate is kept smaller than the estimated mesh size of the ECM. Interestingly, the conjugate provided more specificity for DOX to tumor cells compared to fibroblasts, while free DOX is equally distributed in both tumor and fibroblasts as assessed in the coculture spheroids. Growth inhibition studies show that the released DOX maintains its activity and leads to tumor reduction to the same extent as free DOX. The results obtained here are of relevance for the design of dendrimer-based nanoDDS and for the treatment of solid tumors as they provide critical information regarding desirable surface characteristics and sizes for efficient tumor penetration.
中文翻译:

树枝状大分子共轭可增强阿霉素在细胞外基质表达3D肺癌模型中的肿瘤渗透性和功效。
阿霉素(DOX)是一种化学治疗剂,广泛用于治疗一系列实体瘤。尽管它具有很高的效力,就像许多其他化疗药物一样,但在临床肿瘤学中使用DOX仍存在许多挑战。对于DOX在肺癌中的治疗尤其如此,在体外治疗中,DOX的功效非常高,但是低的肺部分布和脱靶毒性(尤其是心脏毒性)限制了其使用。基于纳米载体的药物递送系统(nanoDDS)已显示出有助于改变生物分布并减轻与DOX相关的脱靶毒性的作用。尽管对实现这些临床益处的设计参数已有深入的了解,但对于能够增强DOX(和其他药物)的肿瘤渗透能力的nanoDDS的设计知之甚少,这是导致DOX疗效下降的另一个主要因素。这项研究的目的是设计一种基于树枝状聚合物的nanoDDS,能够增强在体外3D肺肿瘤模型中测得的DOX的穿透力,并将这些结果与其功效相关联。由A549人肺腺癌细胞/鼠成纤维细胞系(NIH / 3T3细胞系)形成的球体显示出细胞外基质(ECM)的基本成分,这被称为阻碍DOX转运的物理屏障。通过酶促四肽(G4SA-GFLG-DOX)将DOX与第4代琥珀酸封端的聚(酰胺基胺)(PAMAM)树状大分子(G4SA)偶联,得到一个纳米DDS,表面约-17 mV,约5.5 DOX (ζ)电位和10 nm的流体动力学直径(HD)。与自由DOX相比,就DOX荧光而言,确定DOX向球体核心的渗透率是自由DOX的3.1倍,而自由DOX与Caspase 3/7分析法测定的增强功效呈正相关。通过在缀合时屏蔽DOX的质子化胺可最大程度地降低G4SA-GFLG-DOX与高负电荷ECM之间的相互作用,从而提高穿透力,并使缀合物的HD保持小于ECM的估计筛孔尺寸。有趣的是,与成纤维细胞相比,缀合物为肿瘤细胞提供了对DOX更高的特异性,而在共培养球体中,游离的DOX在肿瘤和成纤维细胞中均等分布。生长抑制研究表明,释放的DOX可以保持其活性,并导致肿瘤的减少程度与游离DOX相同。
更新日期:2020-03-31
中文翻译:

树枝状大分子共轭可增强阿霉素在细胞外基质表达3D肺癌模型中的肿瘤渗透性和功效。
阿霉素(DOX)是一种化学治疗剂,广泛用于治疗一系列实体瘤。尽管它具有很高的效力,就像许多其他化疗药物一样,但在临床肿瘤学中使用DOX仍存在许多挑战。对于DOX在肺癌中的治疗尤其如此,在体外治疗中,DOX的功效非常高,但是低的肺部分布和脱靶毒性(尤其是心脏毒性)限制了其使用。基于纳米载体的药物递送系统(nanoDDS)已显示出有助于改变生物分布并减轻与DOX相关的脱靶毒性的作用。尽管对实现这些临床益处的设计参数已有深入的了解,但对于能够增强DOX(和其他药物)的肿瘤渗透能力的nanoDDS的设计知之甚少,这是导致DOX疗效下降的另一个主要因素。这项研究的目的是设计一种基于树枝状聚合物的nanoDDS,能够增强在体外3D肺肿瘤模型中测得的DOX的穿透力,并将这些结果与其功效相关联。由A549人肺腺癌细胞/鼠成纤维细胞系(NIH / 3T3细胞系)形成的球体显示出细胞外基质(ECM)的基本成分,这被称为阻碍DOX转运的物理屏障。通过酶促四肽(G4SA-GFLG-DOX)将DOX与第4代琥珀酸封端的聚(酰胺基胺)(PAMAM)树状大分子(G4SA)偶联,得到一个纳米DDS,表面约-17 mV,约5.5 DOX (ζ)电位和10 nm的流体动力学直径(HD)。与自由DOX相比,就DOX荧光而言,确定DOX向球体核心的渗透率是自由DOX的3.1倍,而自由DOX与Caspase 3/7分析法测定的增强功效呈正相关。通过在缀合时屏蔽DOX的质子化胺可最大程度地降低G4SA-GFLG-DOX与高负电荷ECM之间的相互作用,从而提高穿透力,并使缀合物的HD保持小于ECM的估计筛孔尺寸。有趣的是,与成纤维细胞相比,缀合物为肿瘤细胞提供了对DOX更高的特异性,而在共培养球体中,游离的DOX在肿瘤和成纤维细胞中均等分布。生长抑制研究表明,释放的DOX可以保持其活性,并导致肿瘤的减少程度与游离DOX相同。











































 京公网安备 11010802027423号
京公网安备 11010802027423号