当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Homogeneous Electrochemical Water Oxidation at Neutral pH by Water-Soluble NiII Complexes Bearing Redox Non-innocent Tetraamido Macrocyclic Ligands.
ChemSusChem ( IF 7.5 ) Pub Date : 2020-03-31 , DOI: 10.1002/cssc.202000153 Husileng Lee 1 , Xiujuan Wu 1 , Licheng Sun 1, 2, 3
ChemSusChem ( IF 7.5 ) Pub Date : 2020-03-31 , DOI: 10.1002/cssc.202000153 Husileng Lee 1 , Xiujuan Wu 1 , Licheng Sun 1, 2, 3
Affiliation
|
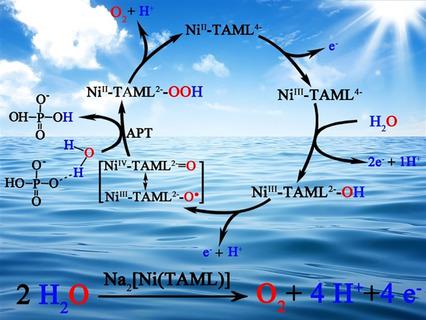
Water oxidation is the bottleneck reaction in artificial photosynthesis. Exploring highly active and stable molecular water oxidation catalysts (WOCs) is still a great challenge. In this study, a water‐soluble NiII complex bearing a redox non‐innocent tetraamido macrocyclic ligand (TAML) is found to be an efficient electrocatalyst for water oxidation in neutral potassium phosphate buffer. Controlled‐potential electrolysis experiments show that it can sustain at a steady current of approximately 0.2 mA cm−2 for >7 h at 1.75 V versus normal hydrogen electrode (NHE) without the formation of NiOx . Electrochemical and spectroelectrochemical tests show that the redox‐active ligand, as well as HPO42− in the buffer, participate in the catalytic cycle. More importantly, catalytically active intermediate [NiIII(TAML2−)−O.] is formed via several proton‐coupled electron transfer processes and reacts with H2O with the assistance of base to release molecular oxygen. Thus, the employment of redox non‐innocent ligands is a useful strategy for designing effective molecular WOCs.
中文翻译:

带有氧化还原非清纯四酰胺基大环配体的水溶性NiII络合物在中性pH下进行均相电化学水氧化。
水的氧化是人工光合作用的瓶颈反应。探索高活性和稳定的分子水氧化催化剂(WOC)仍然是一个巨大的挑战。在这项研究中,发现带有氧化还原非纯四酰胺基大环配体(TAML)的水溶性Ni II络合物是中性磷酸钾缓冲液中水氧化的有效电催化剂。受控电位电解实验表明,与正常的氢电极(NHE)相比,它在1.75 V的稳定电流下,在约0.2 mA cm -2的恒定电流下可维持> 7 h的时间,而不会形成NiO x。电化学和光谱电化学测试表明,氧化还原活性配体以及HPO 4 2-在缓冲液中,参与催化循环。更重要的是,具有催化活性的中间体[Ni III(TAML 2-)-O 。]是通过几个质子耦合的电子转移过程形成的,并在碱的帮助下与H 2 O反应释放出分子氧。因此,采用氧化还原非纯配体是设计有效分子WOC的有用策略。
更新日期:2020-03-31
中文翻译:

带有氧化还原非清纯四酰胺基大环配体的水溶性NiII络合物在中性pH下进行均相电化学水氧化。
水的氧化是人工光合作用的瓶颈反应。探索高活性和稳定的分子水氧化催化剂(WOC)仍然是一个巨大的挑战。在这项研究中,发现带有氧化还原非纯四酰胺基大环配体(TAML)的水溶性Ni II络合物是中性磷酸钾缓冲液中水氧化的有效电催化剂。受控电位电解实验表明,与正常的氢电极(NHE)相比,它在1.75 V的稳定电流下,在约0.2 mA cm -2的恒定电流下可维持> 7 h的时间,而不会形成NiO x。电化学和光谱电化学测试表明,氧化还原活性配体以及HPO 4 2-在缓冲液中,参与催化循环。更重要的是,具有催化活性的中间体[Ni III(TAML 2-)-O 。]是通过几个质子耦合的电子转移过程形成的,并在碱的帮助下与H 2 O反应释放出分子氧。因此,采用氧化还原非纯配体是设计有效分子WOC的有用策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号