当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics of cooperative CO2 adsorption in diamine-appended variants of the metal–organic framework Mg2(dobpdc)
Chemical Science ( IF 7.6 ) Pub Date : 2020-03-31 , DOI: 10.1039/d0sc01087a Jeffrey D. Martell 1, 2, 3, 4 , Phillip J. Milner 1, 2, 3, 4 , Rebecca L. Siegelman 1, 2, 3, 4 , Jeffrey R. Long 1, 2, 3, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2020-03-31 , DOI: 10.1039/d0sc01087a Jeffrey D. Martell 1, 2, 3, 4 , Phillip J. Milner 1, 2, 3, 4 , Rebecca L. Siegelman 1, 2, 3, 4 , Jeffrey R. Long 1, 2, 3, 4, 5
Affiliation
|
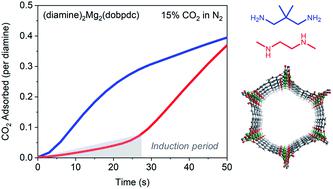
Carbon capture and sequestration is a key element of global initiatives to minimize anthropogenic greenhouse gas emissions. Although many investigations of new candidate CO2 capture materials focus on equilibrium adsorption properties, it is also critical to consider adsorption/desorption kinetics when evaluating adsorbent performance. Diamine-appended variants of the metal–organic framework Mg2(dobpdc) (dobpdc4− = 4,4′-dioxidobiphenyl-3,3′-dicarboxylate) are promising materials for CO2 capture because of their cooperative chemisorption mechanism and associated step-shaped equilibrium isotherms, which enable large working capacities to be accessed with small temperature swings. However, the adsorption/desorption kinetics of these unique materials remain understudied. More generally, despite the necessity of kinetics characterization to advance adsorbents toward commercial separations, detailed kinetic studies of metal–organic framework-based gas separations remain rare. Here, we systematically investigate the CO2 adsorption kinetics of diamine-appended Mg2(dobpdc) variants using a thermogravimetric analysis (TGA) assay. In particular, we examine the effects of diamine structure, temperature, and partial pressure on CO2 adsorption and desorption kinetics. Importantly, most diamine-appended Mg2(dobpdc) variants exhibit an induction period prior to reaching the maximum rate of CO2 adsorption, which we attribute to their unique cooperative chemisorption mechanism. In addition, these materials exhibit inverse Arrhenius behavior, displaying faster adsorption kinetics and shorter induction periods at lower temperatures. Using the Avrami model for nucleation and growth kinetics, we determine rate constants for CO2 adsorption and quantitatively compare rate constants among different diamine-appended variants. Overall, these results provide guidelines for optimizing adsorbent design to facilitate CO2 capture from diverse target streams and highlight kinetic phenomena relevant for other materials in which cooperative chemisorption mechanisms are operative.
中文翻译:

金属-有机骨架Mg2(dobpdc)的二胺附加变体中协同CO2吸附的动力学
碳捕集与封存是减少人为温室气体排放的全球举措的关键要素。尽管对新的候选CO 2捕集材料的许多研究都集中在平衡吸附性能上,但在评估吸附剂性能时考虑吸附/解吸动力学也至关重要。金属-有机骨架Mg 2(dobpdc)(dobpdc 4- = 4,4'-dioxidobiphenyl-3,3'-dicarboxylate)的二胺附加变体是有前途的CO 2材料由于它们具有协同的化学吸附机理和相关的阶梯形平衡等温线,因此可以在较小的温度波动下获得较大的工作能力,从而实现捕集。但是,这些独特的材料的吸附/解吸动力学仍未得到研究。更一般而言,尽管必须进行动力学表征以使吸附剂向商业分离发展,但基于金属-有机骨架的气体分离的详细动力学研究仍然很少。在这里,我们使用热重分析(TGA)分析系统地研究了添加二胺的Mg 2(dobpdc)变体的CO 2吸附动力学。特别是,我们研究了二胺结构,温度和分压对CO 2的影响吸附和解吸动力学。重要的是,大多数添加二胺的Mg 2(dobpdc)变体在达到最大CO 2吸附速率之前均表现出诱导期,这归因于其独特的协同化学吸附机理。另外,这些材料表现出相反的阿累尼乌斯行为,在较低温度下显示出更快的吸附动力学和更短的诱导时间。使用用于成核和生长动力学的Avrami模型,我们确定了CO 2吸附的速率常数,并定量比较了不同的二胺附加变体之间的速率常数。总体而言,这些结果为优化吸附剂设计以促进CO 2提供了指导。 从不同的目标流中捕获并突出显示与其他具有协同化学吸附机制的材料有关的动力学现象。
更新日期:2020-03-31
中文翻译:

金属-有机骨架Mg2(dobpdc)的二胺附加变体中协同CO2吸附的动力学
碳捕集与封存是减少人为温室气体排放的全球举措的关键要素。尽管对新的候选CO 2捕集材料的许多研究都集中在平衡吸附性能上,但在评估吸附剂性能时考虑吸附/解吸动力学也至关重要。金属-有机骨架Mg 2(dobpdc)(dobpdc 4- = 4,4'-dioxidobiphenyl-3,3'-dicarboxylate)的二胺附加变体是有前途的CO 2材料由于它们具有协同的化学吸附机理和相关的阶梯形平衡等温线,因此可以在较小的温度波动下获得较大的工作能力,从而实现捕集。但是,这些独特的材料的吸附/解吸动力学仍未得到研究。更一般而言,尽管必须进行动力学表征以使吸附剂向商业分离发展,但基于金属-有机骨架的气体分离的详细动力学研究仍然很少。在这里,我们使用热重分析(TGA)分析系统地研究了添加二胺的Mg 2(dobpdc)变体的CO 2吸附动力学。特别是,我们研究了二胺结构,温度和分压对CO 2的影响吸附和解吸动力学。重要的是,大多数添加二胺的Mg 2(dobpdc)变体在达到最大CO 2吸附速率之前均表现出诱导期,这归因于其独特的协同化学吸附机理。另外,这些材料表现出相反的阿累尼乌斯行为,在较低温度下显示出更快的吸附动力学和更短的诱导时间。使用用于成核和生长动力学的Avrami模型,我们确定了CO 2吸附的速率常数,并定量比较了不同的二胺附加变体之间的速率常数。总体而言,这些结果为优化吸附剂设计以促进CO 2提供了指导。 从不同的目标流中捕获并突出显示与其他具有协同化学吸附机制的材料有关的动力学现象。











































 京公网安备 11010802027423号
京公网安备 11010802027423号