Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Integrative analysis of genomic amplification-dependent expression and loss-of-function screen identifies ASAP1 as a driver gene in triple-negative breast cancer progression.
Oncogene ( IF 8 ) Pub Date : 2020-03-31 , DOI: 10.1038/s41388-020-1279-3 Jichao He 1 , Ronan P McLaughlin 1 , Lambert van der Beek 1 , Sander Canisius 2 , Lodewyk Wessels 2 , Marcel Smid 3 , John W M Martens 3 , John A Foekens 3 , Yinghui Zhang 1 , Bob van de Water 1
Oncogene ( IF 8 ) Pub Date : 2020-03-31 , DOI: 10.1038/s41388-020-1279-3 Jichao He 1 , Ronan P McLaughlin 1 , Lambert van der Beek 1 , Sander Canisius 2 , Lodewyk Wessels 2 , Marcel Smid 3 , John W M Martens 3 , John A Foekens 3 , Yinghui Zhang 1 , Bob van de Water 1
Affiliation
|
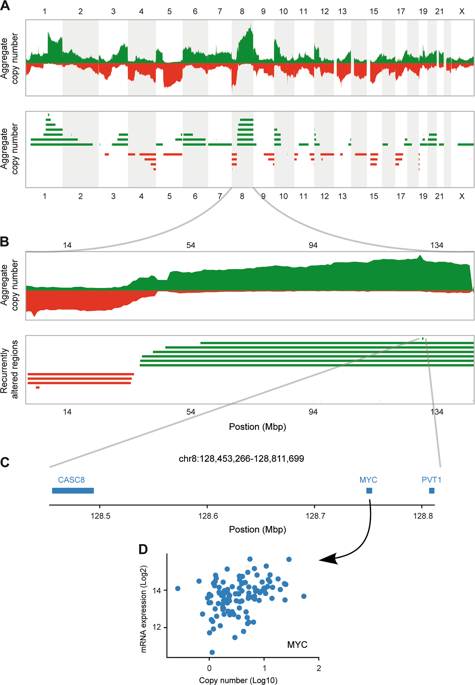
The genetically heterogeneous triple-negative breast cancer (TNBC) continues to be an intractable disease, due to lack of effective targeted therapies. Gene amplification is a major event in tumorigenesis. Genes with amplification-dependent expression are being explored as therapeutic targets for cancer treatment. In this study, we have applied Analytical Multi-scale Identification of Recurring Events analysis and transcript quantification in the TNBC genome across 222 TNBC tumors and identified 138 candidate genes with positive correlation in copy number gain (CNG) and gene expression. siRNA-based loss-of-function screen of the candidate genes has validated EGFR, MYC, ASAP1, IRF2BP2, and CCT5 genes as drivers promoting proliferation in different TNBC cells. MYC, ASAP1, IRF2BP2, and CCT5 display frequent CNG and concurrent expression over 2173 breast cancer tumors (cBioPortal dataset). More frequently are MYC and ASAP1 amplified in TNBC tumors (>30%, n = 320). In particular, high expression of ASAP1, the ADP-ribosylation factor GTPase-activating protein, is significantly related to poor metastatic relapse-free survival of TNBC patients (n = 257, bc-GenExMiner). Furthermore, we have revealed that silencing of ASAP1 modulates numerous cytokine and apoptosis signaling components, such as IL1B, TRAF1, AIFM2, and MAP3K11 that are clinically relevant to survival outcomes of TNBC patients. ASAP1 has been reported to promote invasion and metastasis in various cancer cells. Our findings that ASAP1 is an amplification-dependent TNBC driver gene promoting TNBC cell proliferation, functioning upstream apoptosis components, and correlating to clinical outcomes of TNBC patients, support ASAP1 as a potential actionable target for TNBC treatment.
中文翻译:

对基因组扩增依赖性表达和功能丧失筛选的综合分析确定ASAP1是三阴性乳腺癌进展中的驱动基因。
由于缺乏有效的靶向疗法,遗传异质三阴性乳腺癌(TNBC)仍然是一种顽固性疾病。基因扩增是肿瘤发生中的主要事件。具有扩增依赖性表达的基因正在探索作为癌症治疗的治疗靶标。在这项研究中,我们在222例TNBC肿瘤的TNBC基因组中应用了分析性多尺度重复事件分析和转录本定量,并鉴定了138个候选基因,其拷贝数增加(CNG)和基因表达呈正相关。基于siRNA的候选基因功能丧失筛选已验证EGFR,MYC,ASAP1,IRF2BP2和CCT5基因为促进不同TNBC细胞增殖的驱动器。MYC,ASAP1,IRF2BP2,CCT5和CCT5在2173例乳腺癌肿瘤中显示出频繁的CNG并发表达(cBioPortal数据集)。在TNBC肿瘤中,MYC和ASAP1的扩增频率更高(> 30%,n = 320)。特别是,ASAP-核糖基化因子GTPase激活蛋白ASAP1的高表达与TNBC患者的不良转移无复发生存率显着相关(n = 257,bc-GenExMiner)。此外,我们发现沉默ASAP1会调节许多与TNBC患者的生存结果相关的细胞因子和凋亡信号传导成分,例如IL1B,TRAF1,AIFM2和MAP3K11。据报道,ASAP1可促进多种癌细胞的侵袭和转移。我们的发现ASAP1是一种依赖扩增的TNBC驱动基因,可促进TNBC细胞增殖,并具有上游凋亡成分的功能,
更新日期:2020-03-31
中文翻译:

对基因组扩增依赖性表达和功能丧失筛选的综合分析确定ASAP1是三阴性乳腺癌进展中的驱动基因。
由于缺乏有效的靶向疗法,遗传异质三阴性乳腺癌(TNBC)仍然是一种顽固性疾病。基因扩增是肿瘤发生中的主要事件。具有扩增依赖性表达的基因正在探索作为癌症治疗的治疗靶标。在这项研究中,我们在222例TNBC肿瘤的TNBC基因组中应用了分析性多尺度重复事件分析和转录本定量,并鉴定了138个候选基因,其拷贝数增加(CNG)和基因表达呈正相关。基于siRNA的候选基因功能丧失筛选已验证EGFR,MYC,ASAP1,IRF2BP2和CCT5基因为促进不同TNBC细胞增殖的驱动器。MYC,ASAP1,IRF2BP2,CCT5和CCT5在2173例乳腺癌肿瘤中显示出频繁的CNG并发表达(cBioPortal数据集)。在TNBC肿瘤中,MYC和ASAP1的扩增频率更高(> 30%,n = 320)。特别是,ASAP-核糖基化因子GTPase激活蛋白ASAP1的高表达与TNBC患者的不良转移无复发生存率显着相关(n = 257,bc-GenExMiner)。此外,我们发现沉默ASAP1会调节许多与TNBC患者的生存结果相关的细胞因子和凋亡信号传导成分,例如IL1B,TRAF1,AIFM2和MAP3K11。据报道,ASAP1可促进多种癌细胞的侵袭和转移。我们的发现ASAP1是一种依赖扩增的TNBC驱动基因,可促进TNBC细胞增殖,并具有上游凋亡成分的功能,



























 京公网安备 11010802027423号
京公网安备 11010802027423号