Polymer ( IF 4.1 ) Pub Date : 2020-03-31 , DOI: 10.1016/j.polymer.2020.122428 Chan Hee Lee , Young Chan Bae
|
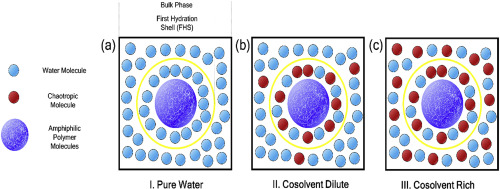
Switching the lower critical solution temperatures (LCSTs) of thermo-sensitive particle gels including poly(N-isopropylacrylamide) (PNIPAM), poly(N-diethylacrylamide) (PNDEAM), and poly(N-vinylcaprolactam) (PNVCL) was investigated in various aqueous solvents. Three prepared particle gels represented a single LCST in a pure water solution near human body temperature. By adding a chaotropic co-solvent, such as dimethylformamide (DMF) or dimethyl sulfoxide (DMSO), to given gels in aqueous solution, the degree of influence on change of LCST was significantly different due to the difference in chemical structures. LCSTs of polymer solutions were affected by changing solvent conditions from pure water to mixed solvents. The LCST behaviors of linear PNIPAM, PNDEAM, and PNVCL in water/DMF or DMSO were determined by thermo-optical analysis (TOA), which supported the switchability of LCSTs in hydrogel systems. We used the photon correlation spectroscopy (PCS) technique to measure the switched LCSTs of hydrogel particles in mixed solvent systems. The switched LCSTs of the investigated systems exhibited a non-linear trend by varying co-solvent contents. A molecular thermodynamic framework, a combination of the modified double lattice with chain length dependence (MDL-CL) model and the Flory-Rehner (F-R) model, was utilized to describe and correlate the liquid-liquid equilibrium (LLE) and thermosensitive swelling behaviors between linear and cross-linked polymer solutions. With the addition of chaotropic co-solvents, a non-linear trend for phase behaviors of the three polymers was described after taking into account temperature and composition dependence on the association fraction () between the species. We assumed the donor-acceptor concept and competitive hydrogen bonding between polymer/water and water/co-solvent. The association fraction for the secondary lattice model was modified from an arbitrarily determined value to a physically meaningful value. Accordingly, the phase equilibrium of the amphiphilic polymers influenced by the structure-breaker characteristics of the co-solute was properly implemented in both experimental and modeling aspects.
中文翻译:

用于在水性溶剂中切换热敏颗粒凝胶的下临界溶液温度的热力学框架
在各种环境下,对包括聚(N-异丙基丙烯酰胺)(PNIPAM),聚(N-二乙基丙烯酰胺)(PNDEAM)和聚(N-乙烯基己内酰胺)(PNVCL)在内的热敏颗粒凝胶的较低临界溶液温度(LCST)进行了切换水性溶剂。三种制备的颗粒凝胶代表在接近人体温度的纯水溶液中的单个LCST。通过将离液型助溶剂(例如二甲基甲酰胺(DMF)或二甲基亚砜(DMSO))添加到水溶液中的给定凝胶中,由于化学结构的不同,对LCST变化的影响程度也显着不同。聚合物溶液的LCST受溶剂条件从纯水变为混合溶剂的影响。通过热光分析(TOA)确定线性PNIPAM,PNDEAM和PNVCL在水/ DMF或DMSO中的LCST行为,支持水凝胶系统中LCST的可切换性。我们使用光子相关光谱技术(PCS)来测量混合溶剂系统中水凝胶颗粒的转换LCST。通过改变助溶剂含量,所研究系统的开关LCST表现出非线性趋势。利用分子热力学框架,结合链长依赖的修饰双晶格模型(MDL-CL)和Flory-Rehner(FR)模型,来描述和关联液-液平衡(LLE)和热敏膨胀行为在线性和交联的聚合物溶液之间。通过添加离液型助溶剂,在考虑了温度和组成对缔合分数的依赖关系之后,描述了三种聚合物的相行为的非线性趋势()之间的物种。我们假设了供体-受体概念以及聚合物/水与水/助溶剂之间的竞争性氢键结合。将次级晶格模型的关联分数从任意确定的值修改为物理上有意义的值。因此,在实验和建模方面都适当地实现了由共溶质的破坏结构特性影响的两亲聚合物的相平衡。











































 京公网安备 11010802027423号
京公网安备 11010802027423号