当前位置:
X-MOL 学术
›
J. Colloid Interface Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Adsorption dynamics of polymeric nanoparticles at an air-water interface with addition of surfactants.
Journal of Colloid and Interface Science ( IF 9.9 ) Pub Date : 2020-03-31 , DOI: 10.1016/j.jcis.2020.03.106 Chang Tian 1 , Jie Feng 2 , Robert K Prud'homme 1
Journal of Colloid and Interface Science ( IF 9.9 ) Pub Date : 2020-03-31 , DOI: 10.1016/j.jcis.2020.03.106 Chang Tian 1 , Jie Feng 2 , Robert K Prud'homme 1
Affiliation
|
HYPOTHESIS
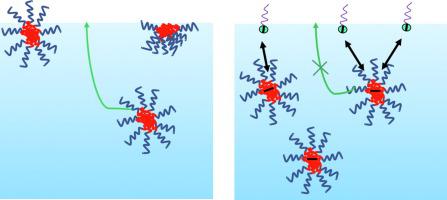
The unusual observation that addition of sodium dodecylsulfate surfactant to an aqueous nanoparticle dispersion slows down the decrease of air:water interfacial tension is attributed to the combined interactions of the nanoparticle with surfactant and surfactant at the air:water interface. Such dynamics are controlled by electrostatic interactions.
EXPERIMENTS
The study of dynamics is achieved using the maximum bubble pressure measurement of surface tension from 0.1 s to 30 s. The NPs are assembled by Flash NanoPrecipitation with 5 kDa polyethylene glycol coronas, and cores of polystyrene, polydimethylsiloxane, or polycaprolactone. Anionic (sodium dodecylsulfate), cationic (cetyltrimethylammonium bromide), and non-ionic (decaethylene glycol monododecyl ether) surfactants are employed over concentration 10-4 to 10-2 mM. The zeta potentials of the NPs are measured with surfactants. Electrostatic repulsion between charged NPs and interface is calculated, as well as the adsorption energy.
FINDINGS
This is the first report to quantitatively explain the effect of surfactants on the dynamics of NP assembly at an interface. An electrostatic energy barrier slows the adsorption kinetics for NPs when the NPs have the same charge as the interface. Increasing ionic strength of the solution reduces the electrostatic barrier. Decreasing interactions between the NP core material and the surfactant reduces the barrier. Our findings offer new insights into understanding of NP interfacial self-assembly dynamics in a complex environment.
中文翻译:

添加表面活性剂后,聚合物纳米颗粒在空气-水界面的吸附动力学。
假设异常的观察结果表明,将十二烷基硫酸钠表面活性剂添加到纳米水分散体中会减慢空气:水界面张力的降低,这归因于纳米颗粒与表面活性剂和表面活性剂在空气:水界面的结合相互作用。这种动力学是通过静电相互作用来控制的。实验使用最大气泡压力测量表面张力(从0.1 s到30 s)来完成动力学研究。NP通过Flash NanoPrecipitation用5 kDa聚乙二醇电晕和聚苯乙烯,聚二甲基硅氧烷或聚己内酯的芯组装。阴离子(十二烷基硫酸钠),阳离子(十六烷基三甲基溴化铵)和非离子(十乙二醇单十二烷基醚)表面活性剂的使用浓度为10-4至10-2 mM。用表面活性剂测量NP的ζ电势。计算带电NP和界面之间的静电排斥力以及吸附能。结果这是第一份定量解释表面活性剂对界面处NP组装动力学的影响的报告。当NP具有与界面相同的电荷时,静电能垒会减慢NP的吸附动力学。溶液离子强度的增加会减小静电势垒。NP核心材料和表面活性剂之间的相互作用降低,从而降低了势垒。我们的发现为在复杂环境中理解NP界面自组装动力学提供了新的见解。以及吸附能 结果这是第一份定量解释表面活性剂对界面处NP组装动力学的影响的报告。当NP具有与界面相同的电荷时,静电能垒会减慢NP的吸附动力学。溶液离子强度的增加会减小静电势垒。NP核心材料和表面活性剂之间的相互作用降低,从而降低了势垒。我们的发现为在复杂环境中理解NP界面自组装动力学提供了新的见解。以及吸附能 结果这是第一份定量解释表面活性剂对界面处NP组装动力学的影响的报告。当NP具有与界面相同的电荷时,静电能垒会减慢NP的吸附动力学。溶液离子强度的增加会减小静电势垒。NP核心材料和表面活性剂之间的相互作用降低,从而降低了势垒。我们的发现为在复杂环境中理解NP界面自组装动力学提供了新的见解。溶液离子强度的增加会减小静电势垒。NP核心材料和表面活性剂之间的相互作用降低,从而降低了势垒。我们的发现为在复杂环境中理解NP界面自组装动力学提供了新的见解。溶液离子强度的增加会减小静电势垒。NP核心材料和表面活性剂之间的相互作用降低,从而降低了势垒。我们的发现为在复杂环境中理解NP界面自组装动力学提供了新的见解。
更新日期:2020-03-31
中文翻译:

添加表面活性剂后,聚合物纳米颗粒在空气-水界面的吸附动力学。
假设异常的观察结果表明,将十二烷基硫酸钠表面活性剂添加到纳米水分散体中会减慢空气:水界面张力的降低,这归因于纳米颗粒与表面活性剂和表面活性剂在空气:水界面的结合相互作用。这种动力学是通过静电相互作用来控制的。实验使用最大气泡压力测量表面张力(从0.1 s到30 s)来完成动力学研究。NP通过Flash NanoPrecipitation用5 kDa聚乙二醇电晕和聚苯乙烯,聚二甲基硅氧烷或聚己内酯的芯组装。阴离子(十二烷基硫酸钠),阳离子(十六烷基三甲基溴化铵)和非离子(十乙二醇单十二烷基醚)表面活性剂的使用浓度为10-4至10-2 mM。用表面活性剂测量NP的ζ电势。计算带电NP和界面之间的静电排斥力以及吸附能。结果这是第一份定量解释表面活性剂对界面处NP组装动力学的影响的报告。当NP具有与界面相同的电荷时,静电能垒会减慢NP的吸附动力学。溶液离子强度的增加会减小静电势垒。NP核心材料和表面活性剂之间的相互作用降低,从而降低了势垒。我们的发现为在复杂环境中理解NP界面自组装动力学提供了新的见解。以及吸附能 结果这是第一份定量解释表面活性剂对界面处NP组装动力学的影响的报告。当NP具有与界面相同的电荷时,静电能垒会减慢NP的吸附动力学。溶液离子强度的增加会减小静电势垒。NP核心材料和表面活性剂之间的相互作用降低,从而降低了势垒。我们的发现为在复杂环境中理解NP界面自组装动力学提供了新的见解。以及吸附能 结果这是第一份定量解释表面活性剂对界面处NP组装动力学的影响的报告。当NP具有与界面相同的电荷时,静电能垒会减慢NP的吸附动力学。溶液离子强度的增加会减小静电势垒。NP核心材料和表面活性剂之间的相互作用降低,从而降低了势垒。我们的发现为在复杂环境中理解NP界面自组装动力学提供了新的见解。溶液离子强度的增加会减小静电势垒。NP核心材料和表面活性剂之间的相互作用降低,从而降低了势垒。我们的发现为在复杂环境中理解NP界面自组装动力学提供了新的见解。溶液离子强度的增加会减小静电势垒。NP核心材料和表面活性剂之间的相互作用降低,从而降低了势垒。我们的发现为在复杂环境中理解NP界面自组装动力学提供了新的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号