当前位置:
X-MOL 学术
›
Mater. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structures, electronic and magnetic properties of the FexN@Cy (x=1–4, y=50, 60, 70) core@shell clusters
Materials Chemistry and Physics ( IF 4.3 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.matchemphys.2020.122959 Zhi Li , Zhen Zhao , Tong-tong Shi
Materials Chemistry and Physics ( IF 4.3 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.matchemphys.2020.122959 Zhi Li , Zhen Zhao , Tong-tong Shi
|
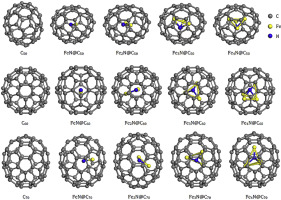
Abstract The configurations, electronic and magnetic properties of the different iron nitride molecules encapsulated C50, C60 and C70 cages have been calculated by using PBE functional. The results show that for the FexN@Cy (x = 1–4, y = 50, 60, 70) core@shell clusters, N atoms prefer to occupy the centers of the Cy (y = 50, 60, 70) cages. As for the FeN@Cy (y = 50, 60, 70) clusters, Fe atoms prefer to approach to the centers of the hexagonal rings. The FexN (x = 1–4) molecules prefer to encapsulated in carbon cages that confirm to the rules: C70>C60>C50. The FeN@C50, Fe2N@C60 and Fe2N@C70 core@shell clusters are more structurally stable than their neighboring. The FexN (x = 1–4) molecules can increase the kinetic stabilities of the Cy (y = 50, 60, 70) cages except for the FeN@C50 clusters. The spin polarization of the FexN (x = 1–4) molecules is significantly decreased by the Cy (y = 50, 60, 70) cages except for the FeN@C60 clusters. All the FexN (x = 1–4) molecules loss electrons to the carbon cages.
中文翻译:

FexN@Cy (x=1–4, y=50, 60, 70) core@shell 簇的结构、电子和磁性特性
摘要 使用 PBE 泛函计算了封装 C50、C60 和 C70 笼的不同氮化铁分子的构型、电子和磁性能。结果表明,对于 FexN@Cy (x = 1–4, y = 50, 60, 70) 核@壳簇,N 原子更喜欢占据 Cy (y = 50, 60, 70) 笼的中心。对于 FeN@Cy (y = 50, 60, 70) 簇,Fe 原子更倾向于靠近六边形环的中心。FexN (x = 1–4) 分子更喜欢封装在符合规则的碳笼中:C70>C60>C50。FeN@C50、Fe2N@C60 和 Fe2N@C70 core@shell 簇比它们的相邻簇在结构上更稳定。除了 FeN@C50 簇之外,FexN (x = 1–4) 分子可以增加 Cy (y = 50, 60, 70) 笼的动力学稳定性。除了 FeN@C60 簇之外,Cy(y = 50、60、70)笼显着降低了 FexN(x = 1-4)分子的自旋极化。所有 FexN (x = 1–4) 分子都失去了碳笼中的电子。
更新日期:2020-07-01
中文翻译:

FexN@Cy (x=1–4, y=50, 60, 70) core@shell 簇的结构、电子和磁性特性
摘要 使用 PBE 泛函计算了封装 C50、C60 和 C70 笼的不同氮化铁分子的构型、电子和磁性能。结果表明,对于 FexN@Cy (x = 1–4, y = 50, 60, 70) 核@壳簇,N 原子更喜欢占据 Cy (y = 50, 60, 70) 笼的中心。对于 FeN@Cy (y = 50, 60, 70) 簇,Fe 原子更倾向于靠近六边形环的中心。FexN (x = 1–4) 分子更喜欢封装在符合规则的碳笼中:C70>C60>C50。FeN@C50、Fe2N@C60 和 Fe2N@C70 core@shell 簇比它们的相邻簇在结构上更稳定。除了 FeN@C50 簇之外,FexN (x = 1–4) 分子可以增加 Cy (y = 50, 60, 70) 笼的动力学稳定性。除了 FeN@C60 簇之外,Cy(y = 50、60、70)笼显着降低了 FexN(x = 1-4)分子的自旋极化。所有 FexN (x = 1–4) 分子都失去了碳笼中的电子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号