iScience ( IF 4.6 ) Pub Date : 2020-03-31 , DOI: 10.1016/j.isci.2020.101028 Ikuko Nagasawa 1 , Masaru Koido 1 , Yuri Tani 1 , Satomi Tsukahara 1 , Kazuhiro Kunimasa 1 , Akihiro Tomida 1
|
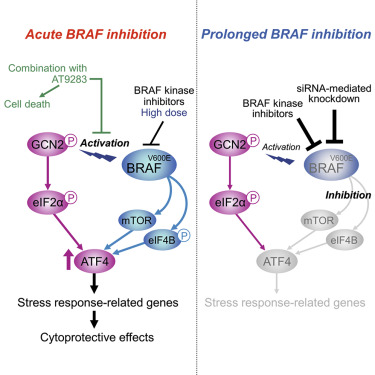
BRAF V600 mutation influences cellular signaling pathways for melanoma development. However, the role of oncogenic BRAF in adaptive stress response pathways is not fully understood. Here, we show that oncogenic BRAF plays an essential role in the induction of ATF4 following the activation of general control non-derepressible 2 (GCN2) kinase during nutrient stress and BRAF-targeted, therapeutic stress. Under GCN2 activation, BRAF ensures ATF4 induction by utilizing mTOR and eIF4B as downstream regulators. In contrast to the MEK-ERK pathway, this signaling pathway remains temporarily active even during treatment with BRAF inhibitors, thereby enabling the transient induction of ATF4. We also identify a chemical compound that prevents BRAF inhibitor-induced activation of the GCN2-ATF4 pathway and produces synergistic cell killing with BRAF inhibitors. Our findings establish a collaborative relationship between oncogenic BRAF and the GCN2-ATF4 signaling pathway, which may provide a novel therapeutic approach to target the adaptive stress response.
中文翻译:

破坏性的ATF4表达机制为针对BRAF的黑色素瘤治疗提供了有效的策略。
宝马V600突变影响黑色素瘤发展的细胞信号通路。然而,致癌BRAF在适应性应激反应途径中的作用尚不完全清楚。在这里,我们显示致癌性BRAF在营养胁迫和以BRAF为靶标的治疗性应激期间激活一般控制不可抑制2(GCN2)激酶后,在ATF4的诱导中起重要作用。在GCN2激活下,BRAF通过使用mTOR和eIF4B作为下游调节剂来确保ATF4诱导。与MEK-ERK途径相反,该信号传导途径即使在用BRAF抑制剂治疗期间也保持暂时活跃,从而能够瞬时诱导ATF4。我们还确定了一种化合物,该化合物可防止BRAF抑制剂诱导的GCN2-ATF4途径激活,并产生与BRAF抑制剂协同杀伤细胞。









































 京公网安备 11010802027423号
京公网安备 11010802027423号