当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Expanding the Medicinal Chemist Toolbox: Comparing Seven C(sp2)–C(sp3) Cross-Coupling Methods by Library Synthesis
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-03-23 , DOI: 10.1021/acsmedchemlett.0c00093 Amanda W. Dombrowski 1 , Nathan J. Gesmundo 1 , Ana L. Aguirre 1 , Katerina A. Sarris 1 , Jonathon M. Young 1 , Andrew R. Bogdan 1 , M. Cynthia Martin 2 , Shasline Gedeon 3 , Ying Wang 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-03-23 , DOI: 10.1021/acsmedchemlett.0c00093 Amanda W. Dombrowski 1 , Nathan J. Gesmundo 1 , Ana L. Aguirre 1 , Katerina A. Sarris 1 , Jonathon M. Young 1 , Andrew R. Bogdan 1 , M. Cynthia Martin 2 , Shasline Gedeon 3 , Ying Wang 1
Affiliation
|
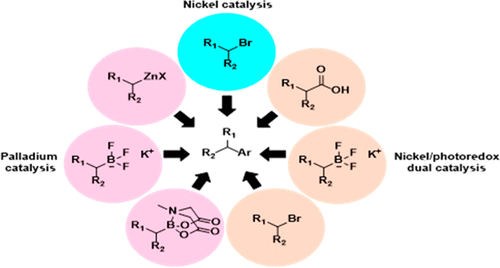
Despite recent advances in the field of C(sp2)–C(sp3) cross-couplings and the accompanying increase in publications, it can be hard to determine which method is appropriate for a given reaction when using the highly functionalized intermediates prevalent in medicinal chemistry. Thus a study was done comparing the ability of seven methods to directly install a diverse set of alkyl groups on “drug-like” aryl structures via parallel library synthesis. Each method showed substrates that it excelled at coupling compared with the other methods. When analyzing the reactions run across all of the methods, a reaction success rate of 50% was achieved. Whereas this is promising, there are still gaps in the scope of direct C(sp2)–C(sp3) coupling methods, like tertiary group installation. The results reported herein should be used to inform future syntheses, assess reaction scope, and encourage medicinal chemists to expand their synthetic toolbox.
中文翻译:

扩展药物化学家工具箱:通过库合成比较七个C(sp 2)–C(sp 3)交叉偶联方法
尽管在C(sp 2)–C(sp 3)交叉偶联领域取得了最新进展,并且随之而来的出版物也有所增加,但是当使用普遍存在的高度官能化的中间体时,很难确定哪种方法适合于给定的反应。药物化学。因此,完成了一项研究,比较了七种方法通过并行库合成在“药物样”芳基结构上直接安装多种烷基的能力。每种方法均显示出与其他方法相比在偶联方面表现出色的底物。分析所有方法中进行的反应时,反应成功率达到50%。尽管这很有希望,但直接C(sp 2)–C(sp 3)的范围仍然存在差距)耦合方法,例如第三组安装。本文报道的结果应用于指导未来的合成,评估反应范围并鼓励药用化学家扩展其合成工具箱。
更新日期:2020-04-23
中文翻译:

扩展药物化学家工具箱:通过库合成比较七个C(sp 2)–C(sp 3)交叉偶联方法
尽管在C(sp 2)–C(sp 3)交叉偶联领域取得了最新进展,并且随之而来的出版物也有所增加,但是当使用普遍存在的高度官能化的中间体时,很难确定哪种方法适合于给定的反应。药物化学。因此,完成了一项研究,比较了七种方法通过并行库合成在“药物样”芳基结构上直接安装多种烷基的能力。每种方法均显示出与其他方法相比在偶联方面表现出色的底物。分析所有方法中进行的反应时,反应成功率达到50%。尽管这很有希望,但直接C(sp 2)–C(sp 3)的范围仍然存在差距)耦合方法,例如第三组安装。本文报道的结果应用于指导未来的合成,评估反应范围并鼓励药用化学家扩展其合成工具箱。











































 京公网安备 11010802027423号
京公网安备 11010802027423号