当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Resolving Membrane Protein-Protein Interactions in Live Cells with Pulsed Interleaved Excitation Fluorescence Cross-Correlation Spectroscopy.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.accounts.9b00625 Shaun Christie 1 , Xiaojun Shi 1 , Adam W Smith 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.accounts.9b00625 Shaun Christie 1 , Xiaojun Shi 1 , Adam W Smith 1
Affiliation
|
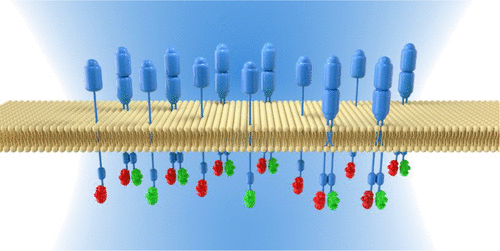
ConspectusThe cell plasma membrane (PM) contains thousands of proteins that sense and respond to the outside environment. These proteins have evolved sensitivity to a wide variety of physical and chemical signals and act as a delivery system across the PM. Membrane proteins are critical for information flow and decision making in the cell and thus are important targets in drug development. A critical aspect of membrane protein function is the way they interact with other proteins, often through the formation of dimers or small oligomers that regulate function at the protein, cell, and organism levels. Resolving membrane protein interactions in a live cell environment is challenging because of the chemical diversity and spatial heterogeneity of the PM. In this Account, we describe a fluorescence technique called pulsed interleaved excitation fluorescence cross-correlation spectroscopy (PIE-FCCS) that is ideally suited to quantify membrane associations in live cells. PIE-FCCS is a two-color fluorescence fluctuation method that can simultaneously measure the concentration, mobility, proximity, and oligomerization state of membrane proteins in situ. It has several advantages over two related approaches, single-molecule tracking (SMT) and Förster resonance energy transfer (FRET), including that it measures all of the properties listed above in a single measurement. Another advantage is that PIE-FCCS is most sensitive at the physiological expression levels for many membrane proteins rather than the very low or high levels typical in other techniques. Here, we review the history of FCCS as it has been applied to study membrane protein interactions in cells. We also describe PIE-FCCS and the advantages it has over biochemical approaches like coimmunoprecipitation (co-IP) and proximity ligation assays (PLA). Finally, we review two classes of membrane proteins that have been studied with FCCS and PIE-FCCS: receptor tyrosine kinases (RTKs) and G protein-coupled receptors (GPCRs). For RTKs, ligand induced dimerization directly regulates the catalytic activity of the kinase, but higher order oligomerization and ligand-independent dimerization can complicate this historically simple paradigm. PIE-FCCS data have resolved a low population of EGFR dimers under basal conditions and assembly into multimers when stimulated with ligand. While GPCRs function primarily as monomers, dimerization has been hypothesized to regulate function for some receptors. PIE-FCCS data have established the dimerization potential of rhodopsin at low densities and were critical for the discovery of a novel dimerization interface in human cone opsins. This Account describes the how FCCS and PIE-FCCS can reveal the details of quaternary interactions in each of these receptor systems.
中文翻译:

用脉冲交错激发荧光互相关光谱法解析活细胞中的膜蛋白相互作用。
结论细胞质膜(PM)包含数以千计的蛋白质,可感知并响应外界环境。这些蛋白质已发展成对多种物理和化学信号的敏感性,并充当整个PM的递送系统。膜蛋白对于细胞中的信息流和决策至关重要,因此是药物开发的重要目标。膜蛋白功能的一个关键方面是它们与其他蛋白相互作用的方式,通常是通过形成在蛋白,细胞和生物体水平上调节功能的二聚体或小的低聚物形成的。由于PM的化学多样性和空间异质性,在活细胞环境中解决膜蛋白相互作用具有挑战性。在这个帐户中,我们描述了一种称为脉冲交错激发荧光互相关光谱(PIE-FCCS)的荧光技术,该技术非常适合量化活细胞中的膜缔合。PIE-FCCS是一种双色荧光波动方法,可以同时测量原位膜蛋白的浓度,迁移率,邻近度和低聚状态。与两种相关方法相比,它具有几个优点,即单分子跟踪(SMT)和福斯特共振能量转移(FRET),包括它可以通过一次测量来测量上面列出的所有特性。另一个优点是,PIE-FCCS在许多膜蛋白的生理表达水平上最敏感,而不是其他技术中典型的非常低或很高的水平。这里,我们回顾了FCCS的历史,因为它已被用于研究细胞中的膜蛋白相互作用。我们还介绍了PIE-FCCS及其与生化方法(如免疫共沉淀(co-IP)和邻近结扎测定(PLA))相比的优势。最后,我们回顾了已经用FCCS和PIE-FCCS研究的两类膜蛋白:受体酪氨酸激酶(RTKs)和G蛋白偶联受体(GPCRs)。对于RTK,配体诱导的二聚化直接调节激酶的催化活性,但更高阶的低聚和不依赖配体的二聚化可使这种历史上简单的范例复杂化。PIE-FCCS数据已解决了在基础条件下少量的EGFR二聚体,并在配体刺激下组装成多聚体的问题。尽管GPCR主要起单体作用,假设二聚化可调节某些受体的功能。PIE-FCCS数据已确定了视紫红质在低密度下的二聚化潜力,对于在人类视锥蛋白中发现新型二聚化界面至关重要。该帐户描述了FCCS和PIE-FCCS如何揭示每个这些受体系统中的四级相互作用的细节。
更新日期:2020-04-23
中文翻译:

用脉冲交错激发荧光互相关光谱法解析活细胞中的膜蛋白相互作用。
结论细胞质膜(PM)包含数以千计的蛋白质,可感知并响应外界环境。这些蛋白质已发展成对多种物理和化学信号的敏感性,并充当整个PM的递送系统。膜蛋白对于细胞中的信息流和决策至关重要,因此是药物开发的重要目标。膜蛋白功能的一个关键方面是它们与其他蛋白相互作用的方式,通常是通过形成在蛋白,细胞和生物体水平上调节功能的二聚体或小的低聚物形成的。由于PM的化学多样性和空间异质性,在活细胞环境中解决膜蛋白相互作用具有挑战性。在这个帐户中,我们描述了一种称为脉冲交错激发荧光互相关光谱(PIE-FCCS)的荧光技术,该技术非常适合量化活细胞中的膜缔合。PIE-FCCS是一种双色荧光波动方法,可以同时测量原位膜蛋白的浓度,迁移率,邻近度和低聚状态。与两种相关方法相比,它具有几个优点,即单分子跟踪(SMT)和福斯特共振能量转移(FRET),包括它可以通过一次测量来测量上面列出的所有特性。另一个优点是,PIE-FCCS在许多膜蛋白的生理表达水平上最敏感,而不是其他技术中典型的非常低或很高的水平。这里,我们回顾了FCCS的历史,因为它已被用于研究细胞中的膜蛋白相互作用。我们还介绍了PIE-FCCS及其与生化方法(如免疫共沉淀(co-IP)和邻近结扎测定(PLA))相比的优势。最后,我们回顾了已经用FCCS和PIE-FCCS研究的两类膜蛋白:受体酪氨酸激酶(RTKs)和G蛋白偶联受体(GPCRs)。对于RTK,配体诱导的二聚化直接调节激酶的催化活性,但更高阶的低聚和不依赖配体的二聚化可使这种历史上简单的范例复杂化。PIE-FCCS数据已解决了在基础条件下少量的EGFR二聚体,并在配体刺激下组装成多聚体的问题。尽管GPCR主要起单体作用,假设二聚化可调节某些受体的功能。PIE-FCCS数据已确定了视紫红质在低密度下的二聚化潜力,对于在人类视锥蛋白中发现新型二聚化界面至关重要。该帐户描述了FCCS和PIE-FCCS如何揭示每个这些受体系统中的四级相互作用的细节。











































 京公网安备 11010802027423号
京公网安备 11010802027423号