当前位置:
X-MOL 学术
›
ACS Appl. Nano Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Roles of Edges and Surfaces of Graphene Oxide in Molecular Recognition of Proteins: Implications for Enzymatic Inhibition of α-Chymotrypsin
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2020-03-30 , DOI: 10.1021/acsanm.0c00543 Subrata Pandit 1 , Mrinmoy De 1
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2020-03-30 , DOI: 10.1021/acsanm.0c00543 Subrata Pandit 1 , Mrinmoy De 1
Affiliation
|
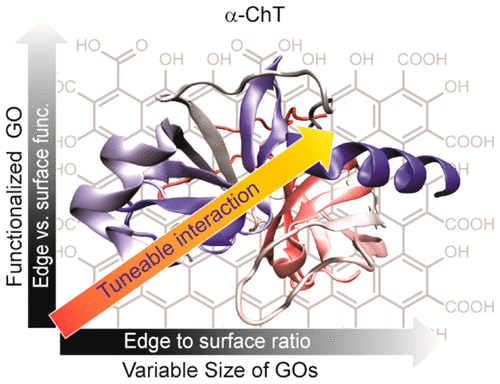
Many biomedical applications of graphene oxide (GO) rely on GO–biomolecular interactions. These interactions can be tuned in two ways: (1) by controlling surface/edge ratio via tuning the lateral size and (2) through selective functionalization of edges or surfaces of GO. To probe this concept, we have prepared four different sizes of GO samples with variable lateral diameters to investigate the effect of surface/edge ratio of GO in protein surface binding. We have also examined the selective functionalization of GO either on edges or surfaces for biomolecular interactions. α-Chymotrypsin was used as a model protein, and activity assays were performed to analyze the role of surface and edges of GO in protein surface interactions. The results show that with increasing size, the inhibitory efficiency of GO was increased, most likely due to an increasing surface-to-charge ratio. We have also observed that surface-functionalized GO shows higher inhibitory efficiency compared to edge-functionalized GO with similar amino acid–based functionality. Furthermore, the activity can be tuned depending on the amino acid side chains of functionalized GO, for which charge and hydrophobicity play the crucial role. Analysis of the kinetics indicates that the inhibition occurred through a reversible competitive binding with no effect on the protein’s secondary structure. This modified GO can be used for other biomedical applications such as sensing, targeted delivery, etc. depending on the alteration of size and functionality or both of GO.
中文翻译:

氧化石墨烯的边缘和表面在蛋白质分子识别中的作用:对α-胰凝乳蛋白酶的酶抑制作用。
氧化石墨烯(GO)的许多生物医学应用都依赖于GO-生物分子相互作用。这些相互作用可以通过两种方式进行调整:(1)通过调整横向尺寸来控制表面/边缘比率,以及(2)通过对GO的边缘或表面进行选择性功能化。为了探讨这一概念,我们准备了四种不同大小的具有可变侧向直径的GO样品,以研究GO的表面/边缘比对蛋白质表面结合的影响。我们还检查了GO在生物分子相互作用的边缘或表面上的选择性功能化。α-胰凝乳蛋白酶用作模型蛋白,并进行活性分析以分析GO的表面和边缘在蛋白表面相互作用中的作用。结果表明,随着粒径的增大,GO的抑制效率增加,最有可能是由于表面电荷比增加。我们还观察到,与具有类似氨基酸功能的边缘官能化GO相比,表面官能化GO表现出更高的抑制效率。此外,可以根据官能化GO的氨基酸侧链来调节活性,为此电荷和疏水性起着至关重要的作用。动力学分析表明抑制作用是通过可逆竞争结合发生的,对蛋白质的二级结构没有影响。根据GO的大小和功能或两者的变化,这种经过改进的GO可以用于其他生物医学应用,例如传感,靶向递送等。我们还观察到,与具有类似氨基酸功能的边缘官能化GO相比,表面官能化GO表现出更高的抑制效率。此外,可以根据官能化GO的氨基酸侧链来调节活性,为此电荷和疏水性起着至关重要的作用。动力学分析表明抑制作用是通过可逆竞争结合发生的,对蛋白质的二级结构没有影响。根据GO的大小和功能或两者的变化,这种经过改进的GO可以用于其他生物医学应用,例如传感,靶向递送等。我们还观察到,与具有类似氨基酸功能的边缘官能化GO相比,表面官能化GO表现出更高的抑制效率。此外,可以根据官能化GO的氨基酸侧链来调节活性,为此电荷和疏水性起着至关重要的作用。动力学分析表明抑制作用是通过可逆竞争结合发生的,对蛋白质的二级结构没有影响。根据GO的大小和功能或两者的变化,这种经过改进的GO可以用于其他生物医学应用,例如传感,靶向递送等。电荷和疏水性起着至关重要的作用。动力学分析表明抑制作用是通过可逆竞争结合发生的,对蛋白质的二级结构没有影响。根据GO的大小和功能或两者的变化,这种经过改进的GO可以用于其他生物医学应用,例如传感,靶向递送等。电荷和疏水性起着至关重要的作用。动力学分析表明抑制作用是通过可逆竞争结合发生的,对蛋白质的二级结构没有影响。根据GO的大小和功能或两者的变化,这种经过改进的GO可以用于其他生物医学应用,例如传感,靶向递送等。
更新日期:2020-03-30
中文翻译:

氧化石墨烯的边缘和表面在蛋白质分子识别中的作用:对α-胰凝乳蛋白酶的酶抑制作用。
氧化石墨烯(GO)的许多生物医学应用都依赖于GO-生物分子相互作用。这些相互作用可以通过两种方式进行调整:(1)通过调整横向尺寸来控制表面/边缘比率,以及(2)通过对GO的边缘或表面进行选择性功能化。为了探讨这一概念,我们准备了四种不同大小的具有可变侧向直径的GO样品,以研究GO的表面/边缘比对蛋白质表面结合的影响。我们还检查了GO在生物分子相互作用的边缘或表面上的选择性功能化。α-胰凝乳蛋白酶用作模型蛋白,并进行活性分析以分析GO的表面和边缘在蛋白表面相互作用中的作用。结果表明,随着粒径的增大,GO的抑制效率增加,最有可能是由于表面电荷比增加。我们还观察到,与具有类似氨基酸功能的边缘官能化GO相比,表面官能化GO表现出更高的抑制效率。此外,可以根据官能化GO的氨基酸侧链来调节活性,为此电荷和疏水性起着至关重要的作用。动力学分析表明抑制作用是通过可逆竞争结合发生的,对蛋白质的二级结构没有影响。根据GO的大小和功能或两者的变化,这种经过改进的GO可以用于其他生物医学应用,例如传感,靶向递送等。我们还观察到,与具有类似氨基酸功能的边缘官能化GO相比,表面官能化GO表现出更高的抑制效率。此外,可以根据官能化GO的氨基酸侧链来调节活性,为此电荷和疏水性起着至关重要的作用。动力学分析表明抑制作用是通过可逆竞争结合发生的,对蛋白质的二级结构没有影响。根据GO的大小和功能或两者的变化,这种经过改进的GO可以用于其他生物医学应用,例如传感,靶向递送等。我们还观察到,与具有类似氨基酸功能的边缘官能化GO相比,表面官能化GO表现出更高的抑制效率。此外,可以根据官能化GO的氨基酸侧链来调节活性,为此电荷和疏水性起着至关重要的作用。动力学分析表明抑制作用是通过可逆竞争结合发生的,对蛋白质的二级结构没有影响。根据GO的大小和功能或两者的变化,这种经过改进的GO可以用于其他生物医学应用,例如传感,靶向递送等。电荷和疏水性起着至关重要的作用。动力学分析表明抑制作用是通过可逆竞争结合发生的,对蛋白质的二级结构没有影响。根据GO的大小和功能或两者的变化,这种经过改进的GO可以用于其他生物医学应用,例如传感,靶向递送等。电荷和疏水性起着至关重要的作用。动力学分析表明抑制作用是通过可逆竞争结合发生的,对蛋白质的二级结构没有影响。根据GO的大小和功能或两者的变化,这种经过改进的GO可以用于其他生物医学应用,例如传感,靶向递送等。











































 京公网安备 11010802027423号
京公网安备 11010802027423号