当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Vanadium-Catalyzed Oxidative Intramolecular Coupling of Tethered Phenols: Formation of Phenol-Dienone Products.
Organic Letters ( IF 4.9 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.orglett.0c00577 Philip H Gilmartin 1 , Marisa C Kozlowski 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.orglett.0c00577 Philip H Gilmartin 1 , Marisa C Kozlowski 1
Affiliation
|
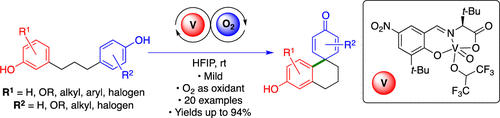
A mild and efficient method for the vanadium-catalyzed intramolecular coupling of tethered free phenols is described. The corresponding phenol-dienone products are prepared directly in good yields with low catalyst loadings. Electronically diverse tethered phenol precursors are well tolerated, and the catalytic method was effectively applied as the key step in syntheses of three natural products and a synthetically useful morphinan alkaloid precursor.
中文翻译:

系留酚的钒催化氧化性分子内偶联:苯酚-二烯酮产物的形成。
描述了一种温和有效的方法,用于钒催化的分子内偶联的游离酚的偶联。相应的苯酚-二烯酮产品直接以高收率和低催化剂负载量制备。电子多样性的束缚酚前体具有良好的耐受性,催化方法有效地用作了合成三种天然产物和可合成的吗啡烷生物碱前体的关键步骤。
更新日期:2020-04-24
中文翻译:

系留酚的钒催化氧化性分子内偶联:苯酚-二烯酮产物的形成。
描述了一种温和有效的方法,用于钒催化的分子内偶联的游离酚的偶联。相应的苯酚-二烯酮产品直接以高收率和低催化剂负载量制备。电子多样性的束缚酚前体具有良好的耐受性,催化方法有效地用作了合成三种天然产物和可合成的吗啡烷生物碱前体的关键步骤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号