当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Allylboration of Ketones and Imines with a Highly Reactive Bifunctional Allyl Pinacolatoboronate Reagent
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.joc.9b03222 Jiaming Liu 1 , Xinbo Tong 1 , Ming Chen 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.joc.9b03222 Jiaming Liu 1 , Xinbo Tong 1 , Ming Chen 1
Affiliation
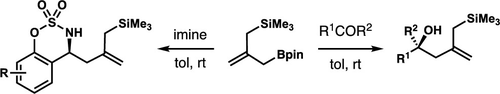
|
Allylboration of ketones and imines with a bifunctional allylboron reagent is reported. Addition of the bifunctional allylboronate to a broad scope of ketones gave tertiary alcohols in excellent yields at ambient temperature without any catalyst or additive. Allylboration of cyclic aldimines also proceeded to give allylated products in good yields under the same reaction conditions. The allylsilane moiety in the product underwent a second allylation with aldehydes or acetals. As such, the bifunctional allylboron reagent serves as a useful linchpin to join two distinct carbonyl compounds to provide synthetically useful intermediates.
中文翻译:

酮和亚胺的高反应性双官能烯丙酸壬酸硼酸硼烷试剂进行烯丙基硼化
报道了用双功能烯丙基硼试剂对酮和亚胺进行烯丙基硼化。将双官能烯丙基硼酸酯加到宽范围的酮中,在室温下以优异的收率得到叔醇,而没有任何催化剂或添加剂。在相同反应条件下,环状亚胺的烯丙基硼化也以高收率进行烯丙基化。产物中的烯丙基硅烷部分与醛或缩醛进行第二烯丙基化。这样,双官能烯丙基硼试剂用作连接两个不同的羰基化合物以提供合成上有用的中间体的有用的关键。
更新日期:2020-04-24
中文翻译:

酮和亚胺的高反应性双官能烯丙酸壬酸硼酸硼烷试剂进行烯丙基硼化
报道了用双功能烯丙基硼试剂对酮和亚胺进行烯丙基硼化。将双官能烯丙基硼酸酯加到宽范围的酮中,在室温下以优异的收率得到叔醇,而没有任何催化剂或添加剂。在相同反应条件下,环状亚胺的烯丙基硼化也以高收率进行烯丙基化。产物中的烯丙基硅烷部分与醛或缩醛进行第二烯丙基化。这样,双官能烯丙基硼试剂用作连接两个不同的羰基化合物以提供合成上有用的中间体的有用的关键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号