当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic, Asymmetric Dearomative Synthesis of Complex Cyclohexanes via a Highly Regio- and Stereoselective Arene Cyclopropanation Using α-Cyanodiazoacetates
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-31 , DOI: 10.1021/jacs.9b13080 Kendrick L. Smith 1 , Cody L. Padgett 1 , William D. Mackay 1 , Jeffrey S. Johnson 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-31 , DOI: 10.1021/jacs.9b13080 Kendrick L. Smith 1 , Cody L. Padgett 1 , William D. Mackay 1 , Jeffrey S. Johnson 1
Affiliation
|
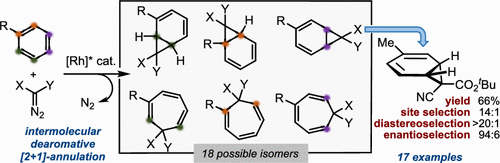
Arene cyclopropanation offers a direct route to higher-order, non-aromatic carbocycles; however, the inherent issue of dictating site selectivity has cumbered the development of novel intermolecular reactions that directly engage the arene pool. This paper describes a highly regio- and stereoselective, Rh2[(S)-PTTL]4-catalyzed arene cyclopropanation using α-cyanodiazoacetates to afford stable norcaradienes bearing three stereogenic centers, one of which is an all-carbon quaternary center. The enantioenriched norcaradienes served as tunable templates for further transformation into stereochemically dense, fused and bicyclic carbocycles containing transmutable functionality.
中文翻译:

使用α-氰基重氮乙酸酯通过高度区域选择性和立体选择性芳烃环丙烷催化不对称脱芳合成复杂环己烷
芳烃环丙烷化提供了一条获得高阶非芳香碳环的直接途径;然而,决定位点选择性的固有问题阻碍了直接参与芳烃池的新型分子间反应的发展。本文描述了一种高度区域选择性和立体选择性、Rh2[(S)-PTTL]4 催化的芳烃环丙烷化反应,使用 α-氰基重氮乙酸酯得到稳定的降卡二烯,其具有三个立体中心,其中一个是全碳四元中心。对映体富集的降卡二烯作为可调节模板,用于进一步转化为立体化学致密、稠合和双环碳环,其中包含可变功能。
更新日期:2020-03-31
中文翻译:

使用α-氰基重氮乙酸酯通过高度区域选择性和立体选择性芳烃环丙烷催化不对称脱芳合成复杂环己烷
芳烃环丙烷化提供了一条获得高阶非芳香碳环的直接途径;然而,决定位点选择性的固有问题阻碍了直接参与芳烃池的新型分子间反应的发展。本文描述了一种高度区域选择性和立体选择性、Rh2[(S)-PTTL]4 催化的芳烃环丙烷化反应,使用 α-氰基重氮乙酸酯得到稳定的降卡二烯,其具有三个立体中心,其中一个是全碳四元中心。对映体富集的降卡二烯作为可调节模板,用于进一步转化为立体化学致密、稠合和双环碳环,其中包含可变功能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号