当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ternary complex formation of AFN-1252 with Acinetobacter baumannii FabI and NADH: Crystallographic and biochemical studies.
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2020-03-30 , DOI: 10.1111/cbdd.13686 Narasimha K Rao 1 , Vijayashankar Nataraj 1 , Mohan Ravi 1 , Love Panchariya 2 , Kirttija Palai 1 , Sumalatha R Talapati 1 , Anirudha Lakshminarasimhan 1 , Murali Ramachandra 1 , Thomas Antony 1
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2020-03-30 , DOI: 10.1111/cbdd.13686 Narasimha K Rao 1 , Vijayashankar Nataraj 1 , Mohan Ravi 1 , Love Panchariya 2 , Kirttija Palai 1 , Sumalatha R Talapati 1 , Anirudha Lakshminarasimhan 1 , Murali Ramachandra 1 , Thomas Antony 1
Affiliation
|
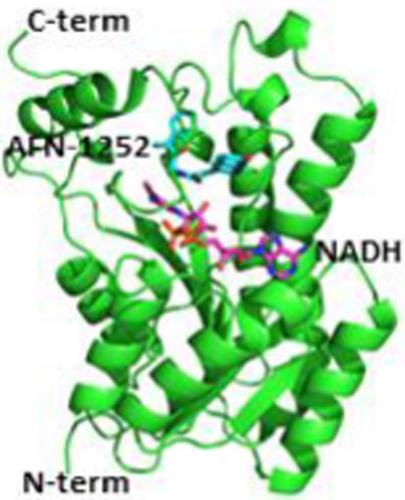
Acinetobacter baumannii is an opportunistic Gram‐negative bacterial pathogen, associated mostly with hospital‐acquired infections. The emergence of drug resistance strains made it necessary to explore new pathways for the development of more effective antibiotics. Enoyl CoA reductase (FabI), a key enzyme in the fatty acid biosynthesis (FAS) pathway, has emerged as a potential target for antibacterial drug development. Earlier reports show that the lead SaFabI inhibitor AFN‐1252 can inhibit FabI from other organisms including Escherichia coli and Burkholderia pseudomallei, but with differential potency. In the present work, we show that AFN‐1252 is a moderate inhibitor of AbFabI with an IC50 of 216 nM. AFN‐1252 stabilized AbFabI with a 4.2°C increase in the melting temperature (Tm) and, interestingly, the stabilization effect was significantly increased in presence of the cofactor NADH (∆Tm = 17°C), suggesting the formation of a ternary complex AbFabI: AFN‐1252: NADH. X‐ray crystallography studies of AbFabI co‐crystalized with AFN‐1252 and NADH confirmed the ternary complex formation. The critical interactions of AFN‐1252 with AbFabI and NADH identified from the co‐crystal structure may facilitate the design and development of new drugs against A. baumannii infections by targeting the FAS pathway.
中文翻译:

AFN-1252与鲍曼不动杆菌FabI和NADH的三元复合物形成:晶体学和生化研究。
鲍曼不动杆菌是机会性革兰氏阴性细菌病原体,主要与医院获得性感染有关。耐药菌株的出现使得有必要探索开发更有效抗生素的新途径。Enoyl CoA还原酶(FabI)是脂肪酸生物合成(FAS)途径中的关键酶,已成为抗菌药物开发的潜在目标。较早的报道表明,SaFabI前导抑制剂AFN-1252可抑制其他生物体中的FabI,包括大肠杆菌和假伯克霍尔德氏菌,但具有不同的效价。在目前的工作中,我们表明AFN-1252是AbFabI的中度抑制剂,IC 50为216 nM。AFN-1252使AbFabI稳定,其熔化温度(T m)提高4.2°C ,有趣的是,在存在辅因子NADH(∆ T m = 17°C)的情况下,稳定作用显着提高,表明形成了三元复合物AbFabI:AFN-1252:NADH。与AFN-1252和NADH共结晶的AbFabI的X射线晶体学研究证实了三元复合物的形成。从共晶体结构中鉴定出的AFN-1252与AbFabI和NADH的关键相互作用可能通过靶向FAS途径促进针对鲍曼不动杆菌感染的新药物的设计和开发。
更新日期:2020-03-30
中文翻译:

AFN-1252与鲍曼不动杆菌FabI和NADH的三元复合物形成:晶体学和生化研究。
鲍曼不动杆菌是机会性革兰氏阴性细菌病原体,主要与医院获得性感染有关。耐药菌株的出现使得有必要探索开发更有效抗生素的新途径。Enoyl CoA还原酶(FabI)是脂肪酸生物合成(FAS)途径中的关键酶,已成为抗菌药物开发的潜在目标。较早的报道表明,SaFabI前导抑制剂AFN-1252可抑制其他生物体中的FabI,包括大肠杆菌和假伯克霍尔德氏菌,但具有不同的效价。在目前的工作中,我们表明AFN-1252是AbFabI的中度抑制剂,IC 50为216 nM。AFN-1252使AbFabI稳定,其熔化温度(T m)提高4.2°C ,有趣的是,在存在辅因子NADH(∆ T m = 17°C)的情况下,稳定作用显着提高,表明形成了三元复合物AbFabI:AFN-1252:NADH。与AFN-1252和NADH共结晶的AbFabI的X射线晶体学研究证实了三元复合物的形成。从共晶体结构中鉴定出的AFN-1252与AbFabI和NADH的关键相互作用可能通过靶向FAS途径促进针对鲍曼不动杆菌感染的新药物的设计和开发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号