当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Expedient Synthesis of Ketones via N‐Heterocyclic Carbene/Nickel‐Catalyzed Redox‐Economical Coupling of Alcohols and Alkynes†
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-03-29 , DOI: 10.1002/cjoc.202000019 Yu‐Qing Li 1 , Feng Li 1 , Shi‐Liang Shi 1
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-03-29 , DOI: 10.1002/cjoc.202000019 Yu‐Qing Li 1 , Feng Li 1 , Shi‐Liang Shi 1
Affiliation
|
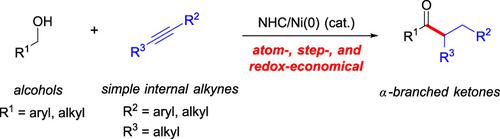
An N‐heterocyclic carbene/nickel‐catalyzed direct coupling of alcohols and internal alkynes to form α‐branched ketones has been developed. This methodology provides a new approach to afford branched ketones, which are difficult to access through the hydroacylation of simple internal alkenes with aldehydes. This redox‐neutral and redox‐economical coupling is free from any oxidative or reductive additives as well as stoichiometric byproducts. These reactions convert both benzylic and aliphatic alcohols and alkynes, two basic feedstock chemicals, into various α‐branched ketones in a single chemical step.
中文翻译:

通过N-杂环卡宾/镍催化的醇和炔烃的氧化还原-经济学偶联合成酮的便捷方法†
已开发出一种N杂环卡宾/镍催化的醇与内部炔烃的直接偶联反应,以形成α支链的酮。这种方法学提供了一种新的方法来提供支链酮,通过简单的内部烯烃与醛的加氢酰化很难获得支链酮。这种氧化还原-中性和氧化还原-经济的偶联不含任何氧化性或还原性添加剂以及化学计量副产物。这些反应可在一个化学步骤中将两种基本原料化学品苄醇,脂肪醇和炔烃转化为各种α支链的酮。
更新日期:2020-03-29
中文翻译:

通过N-杂环卡宾/镍催化的醇和炔烃的氧化还原-经济学偶联合成酮的便捷方法†
已开发出一种N杂环卡宾/镍催化的醇与内部炔烃的直接偶联反应,以形成α支链的酮。这种方法学提供了一种新的方法来提供支链酮,通过简单的内部烯烃与醛的加氢酰化很难获得支链酮。这种氧化还原-中性和氧化还原-经济的偶联不含任何氧化性或还原性添加剂以及化学计量副产物。这些反应可在一个化学步骤中将两种基本原料化学品苄醇,脂肪醇和炔烃转化为各种α支链的酮。











































 京公网安备 11010802027423号
京公网安备 11010802027423号