当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biochemical Characterization of an Arginine 2,3‐Aminomutase with Dual Substrate Specificity
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-03-29 , DOI: 10.1002/cjoc.202000119 Junfeng Zhao 1 , Wenjuan Ji 1 , Xinjian Ji 1 , Qi Zhang 1
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-03-29 , DOI: 10.1002/cjoc.202000119 Junfeng Zhao 1 , Wenjuan Ji 1 , Xinjian Ji 1 , Qi Zhang 1
Affiliation
|
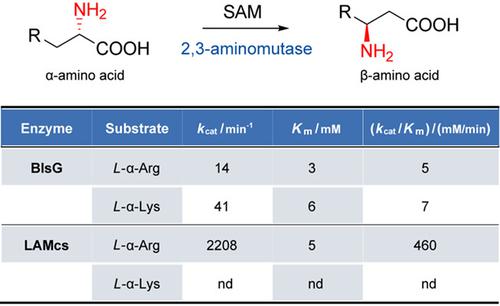
The radical S‐adenosylmethionine (SAM) aminomutases represent an important pathway for the biosynthesis of β‐amino acids. In this study, we report biochemical characterization of BlsG involved in blasticidin S biosynthesis as a radical SAM arginine 2,3‐aminomutase. We showed that BlsG acts on both L‐arginine and L‐lysine with comparable catalytic efficiencies. Similar dual substrate specificity was also observed for the lysine 2,3‐aminomutase from Escherichia coli (LAMEC). The catalytic efficiency of LAMEC is similar to that of BlsG, but is significantly lower than that of the enzyme from Clostridium subterminale (LAMCS), which acts only on L‐lysine rather than on L‐arginine. Moreover, we showed that enzymes can be grouped into two major phylogenetic clades, each corresponding to a certain C3 stereochemistry of the β‐amino acid product. Our study expands the radical SAM aminomutase members and provides insights into enzyme evolution, supporting a trade‐off between substrate promiscuity and catalytic efficiency.
中文翻译:

具有双重底物特异性的精氨酸2,3-氨基变位酶的生化特性
自由基S-腺苷甲硫氨酸(SAM)氨基转移酶是生物合成β-氨基酸的重要途径。在这项研究中,我们报道了参与杀菌素S生物合成的BlsG作为自由基SAM精氨酸2,3-氨基变位酶的生化特性。我们表明,BlsG对L-精氨酸和L-赖氨酸均具有可比的催化效率。对于大肠杆菌(LAM EC)的赖氨酸2,3-氨基变位酶也观察到了相似的双重底物特异性。LAM EC的催化效率与BlsG相似,但是明显低于梭状芽胞杆菌末端酶(LAM CS),后者仅作用于L-赖氨酸而不是L-精氨酸。此外,我们证明了酶可以分为两个主要的系统进化进化枝,每个进化枝对应于β-氨基酸产物的某些C3立体化学。我们的研究扩展了自由基SAM氨基突变酶的成员,并提供了有关酶进化的见解,支持了底物混杂和催化效率之间的权衡。
更新日期:2020-03-29
中文翻译:

具有双重底物特异性的精氨酸2,3-氨基变位酶的生化特性
自由基S-腺苷甲硫氨酸(SAM)氨基转移酶是生物合成β-氨基酸的重要途径。在这项研究中,我们报道了参与杀菌素S生物合成的BlsG作为自由基SAM精氨酸2,3-氨基变位酶的生化特性。我们表明,BlsG对L-精氨酸和L-赖氨酸均具有可比的催化效率。对于大肠杆菌(LAM EC)的赖氨酸2,3-氨基变位酶也观察到了相似的双重底物特异性。LAM EC的催化效率与BlsG相似,但是明显低于梭状芽胞杆菌末端酶(LAM CS),后者仅作用于L-赖氨酸而不是L-精氨酸。此外,我们证明了酶可以分为两个主要的系统进化进化枝,每个进化枝对应于β-氨基酸产物的某些C3立体化学。我们的研究扩展了自由基SAM氨基突变酶的成员,并提供了有关酶进化的见解,支持了底物混杂和催化效率之间的权衡。











































 京公网安备 11010802027423号
京公网安备 11010802027423号