Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Insight into the Confinement Effect of Microporous Carbon in Li/Se Battery Chemistry: A Cathode with Enhanced Conductivity.
Small ( IF 13.0 ) Pub Date : 2020-03-29 , DOI: 10.1002/smll.202000266 Xiwen Wang 1 , Yuqing Tan 1 , Zhixiao Liu 1 , Yuqin Fan 1 , Mingnan Li 1 , Hussein A Younus 1 , Junfei Duan 2 , Huiqiu Deng 3 , Shiguo Zhang 1
Small ( IF 13.0 ) Pub Date : 2020-03-29 , DOI: 10.1002/smll.202000266 Xiwen Wang 1 , Yuqing Tan 1 , Zhixiao Liu 1 , Yuqin Fan 1 , Mingnan Li 1 , Hussein A Younus 1 , Junfei Duan 2 , Huiqiu Deng 3 , Shiguo Zhang 1
Affiliation
|
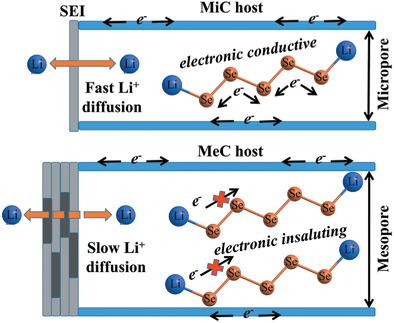
Embedding the fragmented selenium into the micropores of carbon host has been regarded as an effective strategy to change the Li-Se chemistry by a solid-solid mechanism, thereby enabling an excellent cycling stability in Li-Se batteries using carbonate electrolyte. However, the effect of spatial confinement by micropores in the electrochemical behavior of carbon/selenium materials remains ambiguous. A comparative study of using both microporous (MiC) and mesoporous carbons (MeC) with narrow pore size distribution as selenium hosts is herein reported. Systematic investigations reveal that the high Se utilization rate and better electrode kinetics of MiC/Se cathode than MeC/Se cathode may originate from both its improved Li+ and electronic conductivities. The small pore size (<1.35 nm) of the carbon matrices not only facilitates the formation of a compact and robust solid-electrolyte interface (SEI) with low interfacial resistance on cathode, but also alters the insulating nature of Li2 Se due to the emergence of itinerant electrons. By comparing the electrochemical behavior of MiC/Se cathode and the matching relationship between the diameter of pores and the dimension of solvent molecules in carbonate, ether, and solvate ionic liquid electrolyte, the key role of SEI film in the operation of C/Se cathode by quasi-solid-solid mechanism is also highlighted.
中文翻译:

锂/硒电池化学中微孔碳限制作用的新见解:具有增强电导率的阴极。
将碎片化的硒嵌入碳主体的微孔中已被认为是通过固-固机制改变Li-Se化学的有效策略,从而使使用碳酸盐电解质的Li-Se电池具有出色的循环稳定性。然而,在碳/硒材料的电化学行为中,通过微孔进行空间限制的效果仍然不明确。本文报道了同时使用孔径分布窄的微孔碳(MiC)和中孔碳(MeC)作为硒基质的比较研究。系统研究表明,MiC / Se阴极比MeC / Se阴极具有更高的Se利用率和更好的电极动力学,可能源于其改善的Li +和电子电导率。小孔径(<1。35 nm的碳矩阵不仅有助于在阴极上形成具有低界面电阻的紧凑而坚固的固体电解质界面(SEI),而且由于流动电子的出现而改变了Li2 Se的绝缘性质。通过比较MiC / Se阴极的电化学行为以及碳酸盐,乙醚和溶剂化物离子液体电解质中孔直径与溶剂分子尺寸之间的匹配关系,SEI膜在C / Se阴极操作中的关键作用通过准固-固机制也得到了强调。
更新日期:2020-04-22
中文翻译:

锂/硒电池化学中微孔碳限制作用的新见解:具有增强电导率的阴极。
将碎片化的硒嵌入碳主体的微孔中已被认为是通过固-固机制改变Li-Se化学的有效策略,从而使使用碳酸盐电解质的Li-Se电池具有出色的循环稳定性。然而,在碳/硒材料的电化学行为中,通过微孔进行空间限制的效果仍然不明确。本文报道了同时使用孔径分布窄的微孔碳(MiC)和中孔碳(MeC)作为硒基质的比较研究。系统研究表明,MiC / Se阴极比MeC / Se阴极具有更高的Se利用率和更好的电极动力学,可能源于其改善的Li +和电子电导率。小孔径(<1。35 nm的碳矩阵不仅有助于在阴极上形成具有低界面电阻的紧凑而坚固的固体电解质界面(SEI),而且由于流动电子的出现而改变了Li2 Se的绝缘性质。通过比较MiC / Se阴极的电化学行为以及碳酸盐,乙醚和溶剂化物离子液体电解质中孔直径与溶剂分子尺寸之间的匹配关系,SEI膜在C / Se阴极操作中的关键作用通过准固-固机制也得到了强调。









































 京公网安备 11010802027423号
京公网安备 11010802027423号