当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of synthesis pH and EDTA on iron hexacyanoferrate for sodium-ion batteries
Sustainable Energy & Fuels ( IF 5.6 ) Pub Date : 2020-03-30 , DOI: 10.1039/d0se00120a Zachary G. Neale 1, 2, 3, 4 , Chaofeng Liu 1, 2, 3, 4 , Guozhong Cao 1, 2, 3, 4
Sustainable Energy & Fuels ( IF 5.6 ) Pub Date : 2020-03-30 , DOI: 10.1039/d0se00120a Zachary G. Neale 1, 2, 3, 4 , Chaofeng Liu 1, 2, 3, 4 , Guozhong Cao 1, 2, 3, 4
Affiliation
|
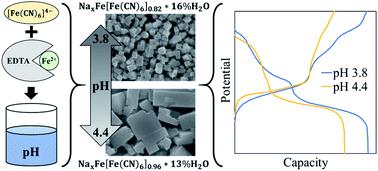
Iron hexacyanoferrate (FeHCF) particles were synthesized at room temperature with ethylenediaminetetraacetic acid (EDTA) at varying pH. The presence of EDTA produced faceted particles and increasing synthesis pH resulted in slower reaction kinetics and larger particles with lower water content and fewer anion vacancies determined by TGA and Mössbauer spectroscopy. Electrochemical testing of sodium metal half cells revealed higher capacity in FeHCF particles grown at lower pH with EDTA, obtaining a maximum discharge capacity of 151 mA h g−1 with 79% capacity retention after 100 cycles at 100 mA g−1 and a rate capability of 122 mA h g−1 at 3.2 A g−1. In contrast, particles grown at higher pH had stunted low-spin Fe redox activity but with improved long-term cyclic stability. These findings demonstrate that small changes in synthesis pH can greatly affect the growth and electrochemical properties of FeHCF when using a pH sensitive chelating agent such as EDTA.
中文翻译:

合成pH和EDTA对钠离子电池六氰合铁酸铁的影响
在室温下用乙二胺四乙酸(EDTA)在不同的pH值下合成六氰合铁酸铁(FeHCF)颗粒。EDTA的存在会产生切面的颗粒,而合成pH值的升高会导致反应动力学变慢,而较大的颗粒则具有较低的水分含量,通过TGA和Mössbauer光谱法测定的阴离子空位较少。钠金属半电池的电化学测试表明,用EDTA在较低pH下生长的FeHCF颗粒具有更高的容量,在100 mA g -1下循环100次后,最大放电容量为151 mA hg -1,容量保持率为79%。 122毫安汞柱-1在3.2克-1。相反,在较高pH下生长的颗粒阻碍了低旋转的Fe氧化还原活性,但具有改善的长期循环稳定性。这些发现表明,当使用pH敏感的螯合剂(如EDTA)时,合成pH的微小变化会极大地影响FeHCF的生长和电化学性能。
更新日期:2020-03-30
中文翻译:

合成pH和EDTA对钠离子电池六氰合铁酸铁的影响
在室温下用乙二胺四乙酸(EDTA)在不同的pH值下合成六氰合铁酸铁(FeHCF)颗粒。EDTA的存在会产生切面的颗粒,而合成pH值的升高会导致反应动力学变慢,而较大的颗粒则具有较低的水分含量,通过TGA和Mössbauer光谱法测定的阴离子空位较少。钠金属半电池的电化学测试表明,用EDTA在较低pH下生长的FeHCF颗粒具有更高的容量,在100 mA g -1下循环100次后,最大放电容量为151 mA hg -1,容量保持率为79%。 122毫安汞柱-1在3.2克-1。相反,在较高pH下生长的颗粒阻碍了低旋转的Fe氧化还原活性,但具有改善的长期循环稳定性。这些发现表明,当使用pH敏感的螯合剂(如EDTA)时,合成pH的微小变化会极大地影响FeHCF的生长和电化学性能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号