Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mapping the redox chemistry of common solvents in solvothermal synthesis through in situ X-ray diffraction
Nanoscale ( IF 5.8 ) Pub Date : 2020-03-30 , DOI: 10.1039/d0nr01240h Nils Lau Nyborg Broge 1, 2, 3, 4 , Frederik Søndergaard-Pedersen 1, 2, 3, 4 , Martin Roelsgaard 1, 2, 3, 4 , Xenia Hassing-Hansen 1, 2, 3, 4 , Bo Brummerstedt Iversen 1, 2, 3, 4
Nanoscale ( IF 5.8 ) Pub Date : 2020-03-30 , DOI: 10.1039/d0nr01240h Nils Lau Nyborg Broge 1, 2, 3, 4 , Frederik Søndergaard-Pedersen 1, 2, 3, 4 , Martin Roelsgaard 1, 2, 3, 4 , Xenia Hassing-Hansen 1, 2, 3, 4 , Bo Brummerstedt Iversen 1, 2, 3, 4
Affiliation
|
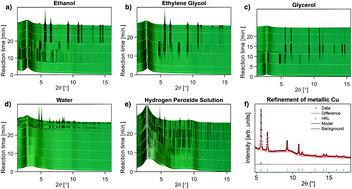
Solvothermal technology shows great promise in “green” materials synthesis, processing, and recycling. The outcome of a specific solvothermal reaction depends strongly on the solvent properties, and the versatility of solvothermal synthesis hinges on the very large changes in solvent properties as a function of temperature and pressure. Here, six simple 3d transition metal nitrate salts (Cu(II), Ni(II), Co(II), Fe(III), Mn(II), Cr(III)) were dissolved in five common solvents (water, ethanol, ethylene glycol, glycerol, and 10% hydrogen peroxide solution) and heated stepwise up to 450 °C at a pressure of 250 bar using an in situ reactor while X-ray scattering data was recorded. A range of crystalline phases were observed in the form of metallic phases, metal oxides, and other ionic compounds. These data by themselves provide simple recipes for synthesis of many technologically important 3d transition metal nanomaterials. However, more generally the oxidation states of the metals in the synthesized materials can be used to map the solvent redox properties under solvothermal conditions. It is found that glycerol and ethylene glycol are strongly reducing, ethanol is moderately reducing, while water is weakly oxidizing. The behavior of the hydrogen peroxide solution is more complex including both oxidization and reduction. Furthermore, it is observed that the reducing powers of ethanol, ethylene glycol, and glycerol are enhanced with increasing temperature. The mapping of the redox properties of these common solvents provides a method for tailoring a given reaction through choice of solvent and reaction temperature. Solvothermal processes represent an environmentally benign alternative to the use of toxic reducing agents in chemical reactions, and quantification of the redox chemistry is a first step in rational materials design.
中文翻译:

通过原位X射线衍射绘制溶剂热合成中常用溶剂的氧化还原化学图
溶剂热技术在“绿色”材料的合成,加工和回收中显示出巨大的希望。特定溶剂热反应的结果在很大程度上取决于溶剂的性质,而溶剂热合成的多功能性取决于溶剂性质随温度和压力的很大变化。在这里,将六种简单的3d过渡金属硝酸盐(Cu(II),Ni(II),Co(II),Fe(III),Mn(II),Cr(III))溶解在五种常见溶剂(水,乙醇)中,乙二醇,甘油和10%的过氧化氢溶液),并使用原位在250 bar的压力下逐步加热至450°C记录X射线散射数据。以金属相,金属氧化物和其他离子化合物的形式观察到一系列结晶相。这些数据本身为合成许多技术上重要的3d过渡金属纳米材料提供了简单的方法。但是,更一般而言,合成材料中金属的氧化态可用于绘制溶剂热条件下的溶剂氧化还原特性。发现甘油和乙二醇强烈还原,乙醇适度还原,而水微弱氧化。过氧化氢溶液的行为更复杂,包括氧化和还原。此外,观察到乙醇,乙二醇和甘油的还原能力随温度升高而增强。这些常用溶剂的氧化还原特性的映射提供了一种通过选择溶剂和反应温度来调整给定反应的方法。溶剂热法是在化学反应中使用有毒还原剂的一种对环境有益的替代方法,氧化还原化学的定量分析是合理材料设计的第一步。
更新日期:2020-04-24
中文翻译:

通过原位X射线衍射绘制溶剂热合成中常用溶剂的氧化还原化学图
溶剂热技术在“绿色”材料的合成,加工和回收中显示出巨大的希望。特定溶剂热反应的结果在很大程度上取决于溶剂的性质,而溶剂热合成的多功能性取决于溶剂性质随温度和压力的很大变化。在这里,将六种简单的3d过渡金属硝酸盐(Cu(II),Ni(II),Co(II),Fe(III),Mn(II),Cr(III))溶解在五种常见溶剂(水,乙醇)中,乙二醇,甘油和10%的过氧化氢溶液),并使用原位在250 bar的压力下逐步加热至450°C记录X射线散射数据。以金属相,金属氧化物和其他离子化合物的形式观察到一系列结晶相。这些数据本身为合成许多技术上重要的3d过渡金属纳米材料提供了简单的方法。但是,更一般而言,合成材料中金属的氧化态可用于绘制溶剂热条件下的溶剂氧化还原特性。发现甘油和乙二醇强烈还原,乙醇适度还原,而水微弱氧化。过氧化氢溶液的行为更复杂,包括氧化和还原。此外,观察到乙醇,乙二醇和甘油的还原能力随温度升高而增强。这些常用溶剂的氧化还原特性的映射提供了一种通过选择溶剂和反应温度来调整给定反应的方法。溶剂热法是在化学反应中使用有毒还原剂的一种对环境有益的替代方法,氧化还原化学的定量分析是合理材料设计的第一步。











































 京公网安备 11010802027423号
京公网安备 11010802027423号