当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Brine rejection and hydrate formation upon freezing of NaCl aqueous solutions
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020/03/30 , DOI: 10.1039/c9cp05436g Ifigeneia Tsironi 1, 2, 3, 4, 5 , Daniel Schlesinger 3, 5, 6, 7 , Alexander Späh 1, 2, 3, 4, 5 , Lars Eriksson 3, 5, 8, 9 , Mo Segad 3, 5, 8, 9 , Fivos Perakis 1, 2, 3, 4, 5
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020/03/30 , DOI: 10.1039/c9cp05436g Ifigeneia Tsironi 1, 2, 3, 4, 5 , Daniel Schlesinger 3, 5, 6, 7 , Alexander Späh 1, 2, 3, 4, 5 , Lars Eriksson 3, 5, 8, 9 , Mo Segad 3, 5, 8, 9 , Fivos Perakis 1, 2, 3, 4, 5
Affiliation
|
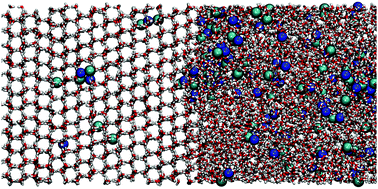
Studying the freezing of saltwater on a molecular level is of fundamental importance for improving freeze desalination techniques. In this study, we investigate the freezing process of NaCl solutions using a combination of X-ray diffraction and molecular dynamics simulations (MD) for different salt-water concentrations, ranging from seawater conditions to saturation. A linear superposition model reproduces well the brine rejection due to hexagonal ice Ih formation and allows us to quantify the fraction of ice and brine. Furthermore, upon cooling at T = 233 K, we observe the formation of NaCl·2H2O hydrates (hydrohalites), which coexist with ice Ih. MD simulations are utilized to model the formation of NaCl crystal hydrates. From the simulations, we estimate that the salinity of the newly produced ice is 0.5% mass percent (m/m) due to ion inclusions, which is within the salinity limits of fresh water. In addition, we show the effect of ions on the local ice structure using the tetrahedrality parameter and follow the crystallite formation using the ion coordination parameter and cluster analysis.
中文翻译:

氯化钠水溶液冷冻后除盐水和形成水合物
在分子水平上研究盐水的冷冻对于改进冷冻淡化技术具有根本的重要性。在这项研究中,我们结合X射线衍射和分子动力学模拟(MD),针对从海水条件到饱和度的不同盐水浓度,研究了NaCl溶液的冷冻过程。线性叠加模型很好地重现了由于六方冰Ih形成而导致的盐水排斥,并允许我们量化冰和盐水的比例。此外,在T = 233 K冷却后,我们观察到NaCl·2H 2的形成。O水合物(卤化氢),与冰Ih共存。MD模拟用于模拟NaCl晶体水合物的形成。通过模拟,我们估计由于离子夹杂,新产生的冰的盐度为0.5%质量百分比(m / m),这在淡水的盐度限制内。此外,我们使用四面度参数显示离子对局部冰结构的影响,并使用离子配位参数和聚类分析跟踪微晶形成。
更新日期:2020-04-08
中文翻译:

氯化钠水溶液冷冻后除盐水和形成水合物
在分子水平上研究盐水的冷冻对于改进冷冻淡化技术具有根本的重要性。在这项研究中,我们结合X射线衍射和分子动力学模拟(MD),针对从海水条件到饱和度的不同盐水浓度,研究了NaCl溶液的冷冻过程。线性叠加模型很好地重现了由于六方冰Ih形成而导致的盐水排斥,并允许我们量化冰和盐水的比例。此外,在T = 233 K冷却后,我们观察到NaCl·2H 2的形成。O水合物(卤化氢),与冰Ih共存。MD模拟用于模拟NaCl晶体水合物的形成。通过模拟,我们估计由于离子夹杂,新产生的冰的盐度为0.5%质量百分比(m / m),这在淡水的盐度限制内。此外,我们使用四面度参数显示离子对局部冰结构的影响,并使用离子配位参数和聚类分析跟踪微晶形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号