当前位置:
X-MOL 学术
›
PLOS Pathog.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of Hepatitis B virus capsid phosphorylation in nucleocapsid disassembly and covalently closed circular DNA formation.
PLoS Pathogens ( IF 5.5 ) Pub Date : 2020-03-30 , DOI: 10.1371/journal.ppat.1008459 Jun Luo 1 , Ji Xi 1 , Lu Gao 2 , Jianming Hu 1
PLoS Pathogens ( IF 5.5 ) Pub Date : 2020-03-30 , DOI: 10.1371/journal.ppat.1008459 Jun Luo 1 , Ji Xi 1 , Lu Gao 2 , Jianming Hu 1
Affiliation
|
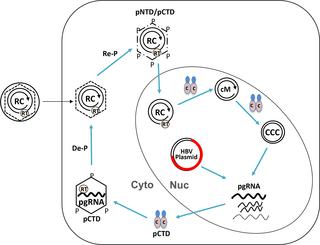
Hepatitis B virus (HBV) delivers a partially double-stranded, relaxed circular (RC) DNA genome in complete virions to the host cell nucleus for conversion to the covalently closed circular (CCC) DNA, which establishes and sustains viral infection. An overlength pregenomic RNA (pgRNA) is then transcribed from CCC DNA and packaged into immature nucleocapsids (NCs) by the viral core (HBc) protein. pgRNA is reverse transcribed to produce RC DNA in mature NCs, which are then enveloped and secreted as complete virions, or delivered to the nucleus to replenish the nuclear CCC DNA pool. RC DNA, whether originating from extracellular virions or intracellular mature NCs, must be released upon NC disassembly (uncoating) for CCC DNA formation. HBc is known to undergo dynamic phosphorylation and dephosphorylation at its C-terminal domain (CTD) to facilitate pgRNA packaging and reverse transcription. Here, two putative phosphorylation sites in the HBc N-terminal domain (NTD), S44 and S49, were targeted for genetic and biochemical analysis to assess their potential roles in viral replication. The NTD mutant that mimics the non-phosphorylated state (N2A) was competent in all steps of viral replication tested from capsid assembly, pgRNA packaging, reverse transcription, to virion secretion, except for a decrease in CCC DNA formation. On the other hand, the phosphor-mimetic mutant N2E showed a defect in the early step of pgRNA packaging but enhanced the late step of mature NC uncoating and consequently, increased CCC DNA formation. N2E also enhanced phosphorylation in CTD and possibly elsewhere in HBc. Furthermore, inhibition of the cyclin-dependent kinase 2 (CDK2), which is packaged into viral capsids, could block CCC DNA formation. These results prompted us to propose a model whereby rephosphorylation of HBc at both NTD and CTD by the packaged CDK2, following CTD dephosphorylation during NC maturation, facilitates uncoating and CCC DNA formation by destabilizing mature NCs.
中文翻译:

乙型肝炎病毒衣壳磷酸化在核衣壳拆卸和共价闭合环状DNA形成中的作用。
乙型肝炎病毒(HBV)将完整病毒粒子中的部分双链,松弛的环状(RC)DNA基因组传递至宿主细胞核,以转化为共价闭合的环状(CCC)DNA,从而建立并维持病毒感染。然后从CCC DNA转录一个超长的基因组RNA(pgRNA),并通过病毒核心(HBc)蛋白包装成未成熟的核衣壳(NCs)。pgRNA被反转录以在成熟的NC中产生RC DNA,然后将其包裹并分泌为完整的病毒体,或递送至细胞核以补充核CCC DNA库。RC DNA,无论是源自细胞外病毒体还是细胞内成熟的NC,都必须在NC拆卸(解涂层)后释放,以形成CCC DNA。已知HBc在其C末端结构域(CTD)处发生动态磷酸化和去磷酸化,以促进pgRNA包装和逆转录。在这里,HBc N末端结构域(NTD)中的两个假定的磷酸化位点S44和S49被用于遗传和生化分析,以评估它们在病毒复制中的潜在作用。模仿非磷酸化状态(N2A)的NTD突变体在病毒复制的所有步骤中均能胜任,从衣壳装配,pgRNA包装,逆转录到病毒体分泌,除CCC DNA形成减少外。另一方面,模拟磷的突变体N2E在pgRNA包装的早期显示出缺陷,但增强了成熟NC脱膜的后期,因此增加了CCC DNA的形成。N2E还增强了CTD和HBc其他地方的磷酸化。此外,抑制包装在病毒衣壳中的细胞周期蛋白依赖性激酶2(CDK2)可能会阻止CCC DNA的形成。这些结果促使我们提出了一个模型,其中在NC成熟过程中CTD去磷酸化后,包装的CDK2在NTD和CTD处对HBc进行再磷酸化,从而通过破坏稳定的NC促进脱膜和CCC DNA的形成。
更新日期:2020-03-30
中文翻译:

乙型肝炎病毒衣壳磷酸化在核衣壳拆卸和共价闭合环状DNA形成中的作用。
乙型肝炎病毒(HBV)将完整病毒粒子中的部分双链,松弛的环状(RC)DNA基因组传递至宿主细胞核,以转化为共价闭合的环状(CCC)DNA,从而建立并维持病毒感染。然后从CCC DNA转录一个超长的基因组RNA(pgRNA),并通过病毒核心(HBc)蛋白包装成未成熟的核衣壳(NCs)。pgRNA被反转录以在成熟的NC中产生RC DNA,然后将其包裹并分泌为完整的病毒体,或递送至细胞核以补充核CCC DNA库。RC DNA,无论是源自细胞外病毒体还是细胞内成熟的NC,都必须在NC拆卸(解涂层)后释放,以形成CCC DNA。已知HBc在其C末端结构域(CTD)处发生动态磷酸化和去磷酸化,以促进pgRNA包装和逆转录。在这里,HBc N末端结构域(NTD)中的两个假定的磷酸化位点S44和S49被用于遗传和生化分析,以评估它们在病毒复制中的潜在作用。模仿非磷酸化状态(N2A)的NTD突变体在病毒复制的所有步骤中均能胜任,从衣壳装配,pgRNA包装,逆转录到病毒体分泌,除CCC DNA形成减少外。另一方面,模拟磷的突变体N2E在pgRNA包装的早期显示出缺陷,但增强了成熟NC脱膜的后期,因此增加了CCC DNA的形成。N2E还增强了CTD和HBc其他地方的磷酸化。此外,抑制包装在病毒衣壳中的细胞周期蛋白依赖性激酶2(CDK2)可能会阻止CCC DNA的形成。这些结果促使我们提出了一个模型,其中在NC成熟过程中CTD去磷酸化后,包装的CDK2在NTD和CTD处对HBc进行再磷酸化,从而通过破坏稳定的NC促进脱膜和CCC DNA的形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号