PLOS ONE ( IF 2.9 ) Pub Date : 2020-03-30 , DOI: 10.1371/journal.pone.0230489 Adhi Kristianto Sugianli 1 , Franciscus Ginting 2 , R Lia Kusumawati 3 , Ida Parwati 1 , Menno D de Jong 4 , Frank van Leth 5, 6 , Constance Schultsz 4, 5, 6
|
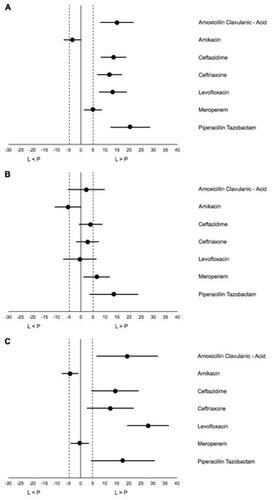
Surveillance of antimicrobial resistance (AMR) enables monitoring of trends in AMR prevalence. WHO recommends laboratory-based surveillance to obtain actionable AMR data at local or national level. However, laboratory-based surveillance may lead to overestimation of the prevalence of AMR due to bias. The objective of this study is to assess the difference in resistance prevalence between laboratory-based and population-based surveillance (PBS) among uropathogens in Indonesia. We included all urine samples submitted to the laboratory growing Escherichia coli and Klebsiella pneumoniae in the laboratory-based surveillance. Population-based surveillance data were collected in a cross-sectional survey of AMR in E. coli and K. pneumoniae isolated from urine samples among consecutive patients with symptoms of UTI, attending outpatient clinics and hospital wards. Data were collected between 1 April 2014 until 31 May 2015. The difference in percentage resistance (95% confidence intervals) between laboratory- and population-based surveillance was calculated for relevant antibiotics. A difference larger than +/- 5 percent points was defined as a biased result, precluding laboratory-based surveillance for guiding empirical treatment. We observed high prevalence of AMR ranging between 63.1% (piperacillin-tazobactam) and 85% (ceftriaxone) in laboratory-based surveillance and 41.3% (piperacillin-tazobactam) and 74.2% (ceftriaxone) in population-based surveillance, except for amikacin and meropenem (5.7%/9.8%; 10.8%/5.9%; [laboratory-/population-based surveillance], respectively). Laboratory-based surveillance yielded significantly higher AMR prevalence estimates than population-based surveillance. This difference was much larger when comparing surveillance data from outpatients than from inpatients. All point estimates of the difference between the two surveillance systems were larger than 5 percent points, except for amikacin and meropenem. Laboratory-based AMR surveillance of uropathogens, is not adequate to guide empirical treatment for community-based settings in Indonesia.
中文翻译:

基于实验室与基于人群的抗菌药物耐药性监测,为印度尼西亚疑似尿路感染的经验治疗提供信息。
抗菌素耐药性 (AMR) 监测可以监测 AMR 流行趋势。世卫组织建议开展基于实验室的监测,以获得地方或国家层面可采取行动的抗菌素耐药性数据。然而,基于实验室的监测可能会因偏差而导致对 AMR 患病率的高估。本研究的目的是评估印度尼西亚尿路病原体实验室监测和人群监测 (PBS) 耐药率的差异。我们将提交给大肠杆菌和肺炎克雷伯菌实验室的所有尿液样本纳入基于实验室的监测。基于人群的监测数据是在大肠杆菌AMR 横断面调查中收集的。大肠杆菌和K . 从连续出现尿路感染症状、到门诊和医院病房就诊的患者的尿液样本中分离出肺炎链球菌。数据收集时间为2014年4月1日至2015年5月31日。计算了相关抗生素的实验室监测和人群监测之间的耐药百分比差异(95%置信区间)。大于+/-5%的差异被定义为有偏差的结果,排除了基于实验室的监测来指导经验性治疗。我们观察到,在基于实验室的监测中,AMR 的患病率很高,范围在 63.1%(哌拉西林-他唑巴坦)和 85%(头孢曲松)之间;在基于人群的监测中,AMR 患病率在 41.3%(哌拉西林-他唑巴坦)和 74.2%(头孢曲松)之间,阿米卡星和阿米卡星除外。美罗培南(分别为 5.7%/9.8%;10.8%/5.9%;[基于实验室/人群的监测])。基于实验室的监测得出的 AMR 患病率估计值明显高于基于人群的监测。当比较门诊患者的监测数据时,这种差异比住院患者的监测数据要大得多。除阿米卡星和美罗培南外,两个监测系统之间差异的所有点估计均大于 5%。基于实验室的尿路病原体 AMR 监测不足以指导印度尼西亚社区环境的经验治疗。











































 京公网安备 11010802027423号
京公网安备 11010802027423号