Nature Structural & Molecular Biology ( IF 12.5 ) Pub Date : 2020-03-30 , DOI: 10.1038/s41594-020-0401-0 Zengqin Deng 1, 2 , Zhihui He 1, 2 , Grigory Maksaev 1, 2 , Ryan M Bitter 1, 2 , Michael Rau 3 , James A J Fitzpatrick 1, 3, 4, 5 , Peng Yuan 1, 2
|
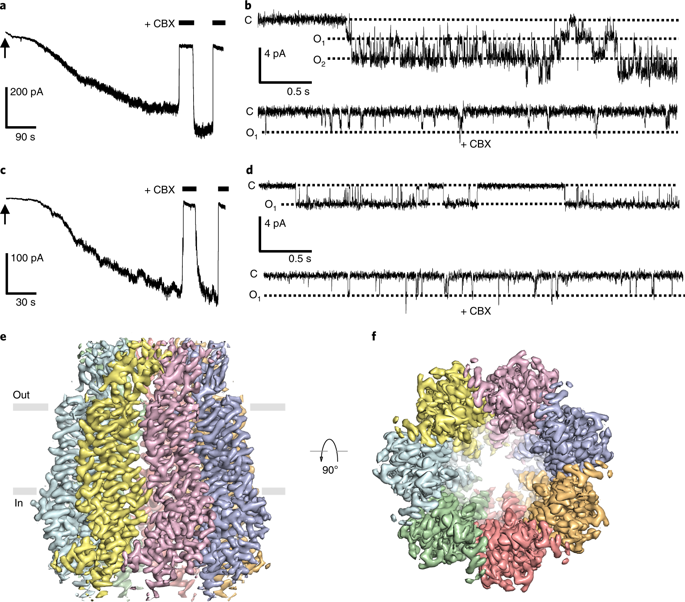
The plasma membrane adenosine triphosphate (ATP) release channel pannexin 1 (PANX1) has been implicated in many physiological and pathophysiological processes associated with purinergic signaling, including cancer progression, apoptotic cell clearance, inflammation, blood pressure regulation, oocyte development, epilepsy and neuropathic pain. Here we present near-atomic-resolution structures of human and frog PANX1 determined by cryo-electron microscopy that revealed a heptameric channel architecture. Compatible with ATP permeation, the transmembrane pore and cytoplasmic vestibule were exceptionally wide. An extracellular tryptophan ring located at the outer pore created a constriction site, potentially functioning as a molecular sieve that restricts the size of permeable substrates. The amino and carboxyl termini, not resolved in the density map, appeared to be structurally dynamic and might contribute to narrowing of the pore during channel gating. In combination with functional characterization, this work elucidates the previously unknown architecture of pannexin channels and establishes a foundation for understanding their unique channel properties.
中文翻译:

ATP释放通道pannexin 1。
质膜三磷酸腺苷(ATP)释放通道pannexin 1(PANX1)已参与嘌呤能信号传导的许多生理和病理生理过程,包括癌症进展,凋亡细胞清除,炎症,血压调节,卵母细胞发育,癫痫和神经性疼痛。在这里,我们介绍了人类和青蛙PANX1的近原子分辨率结构,该结构由冷冻电子显微镜确定,揭示了七聚体通道结构。与ATP渗透相容,跨膜孔和胞质前庭异常宽。位于外孔的细胞外色氨酸环产生一个缩窄位点,可能充当限制可渗透底物尺寸的分子筛。在密度图中未解析的氨基和羧基末端,似乎在结构上是动态的,并且可能在通道门控期间导致孔变窄。结合功能表征,这项工作阐明了Pannexin通道的先前未知架构,并为理解其独特通道特性奠定了基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号