British Journal of Cancer ( IF 6.4 ) Pub Date : 2020-03-31 , DOI: 10.1038/s41416-020-0816-8 Eva Blondeaux 1 , Matteo Lambertini 2, 3 , Andrea Michelotti 4 , Benedetta Conte 1 , Marco Benasso 5 , Chiara Dellepiane 1 , Claudia Bighin 1 , Simona Pastorino 1 , Alessia Levaggi 2 , Alessia D' Alonzo 1 , Francesca Poggio 1 , Giulia Buzzatti 1 , Chiara Molinelli 1 , Piero Fregatti 6 , Sergio Bertoglio 7, 8 , Francesco Boccardo 2, 3 , Lucia Del Mastro 1, 3
|
Background
Adjuvant chemotherapy is the standard of care in high-risk early breast cancer patients. Dose-dense should be the preferred schedule of administration. However, its long-term benefit is unknown.
Methods
In the Italian multicentre Phase 3 randomised MIG-1 trial, node-positive and high-risk node- negative breast cancer patients were randomised to receive six cycles of adjuvant fluorouracil, epirubicin and cyclophosphamide regimen administered every 3 (FEC21) or 2 (FEC14) weeks. The primary endpoint was overall survival (OS), and the secondary endpoint was event-free survival (EFS).
Results
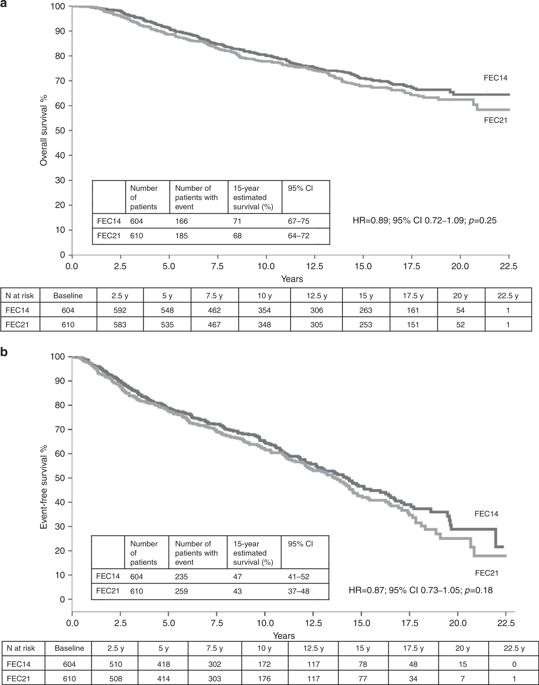
From 1992 to 1997, 1214 patients were included. Median follow-up was 15.8 years. In all, 15-year OS was 71% and 68% in the FEC14 and FEC21 groups, respectively (HR = 0.89; p = 0.25). In all, 15-year EFS was 47% and 43% in the FEC14 and FEC21 groups, respectively (HR = 0.87; p = 0.18). In a pre-planned subgroup analysis, among patients with hormone receptor-negative tumours, 15-year OS was 70% and 65% in the FEC14 and FEC21 groups, respectively (HR = 0.73; 95% CI: 0.51–1.06); 15-year EFS was 58% and 43% in the FEC14 and FEC21 groups, respectively (HR = 0.70; 95% CI: 0.51–0.96).
Conclusions
Updated results from the MIG-1 study are numerically in favour of dose-dense chemotherapy, and suggest a long-term benefit of this approach in high-risk early breast cancer patients.
中文翻译:

早期乳腺癌患者的剂量密集辅助化疗:3期Mammella InterGruppo(MIG)-1研究的15年结果。
背景
辅助化疗是高危早期乳腺癌患者的治疗标准。剂量密集应该是首选的给药方案。但是,其长期利益尚不清楚。
方法
在意大利的多中心3期MIG-1随机试验中,淋巴结阳性和高风险淋巴结阴性的乳腺癌患者被随机分配接受六个周期的辅助氟尿嘧啶,表柔比星和环磷酰胺治疗方案,每3次(FEC21)或2次(FEC14)周。主要终点是总体生存期(OS),次要终点是无事件生存期(EFS)。
结果
从1992年到1997年,共纳入1214例患者。中位随访时间为15.8年。总体而言,FEC14和FEC21组的15年OS分别为71%和68%(HR = 0.89;p = 0.25)。总体而言,FEC14和FEC21组的15年EFS分别为47%和43%(HR = 0.87;p = 0.18)。在一项预先计划的亚组分析中,在FEC14和FEC21组中,荷尔蒙受体阴性肿瘤患者的15年OS分别为70%和65%(HR = 0.73; 95%CI:0.51-1.06);FEC14和FEC21组的15年EFS分别为58%和43%(HR = 0.70; 95%CI:0.51-0.96)。
结论
MIG-1研究的最新结果在数值上支持剂量密集化学疗法,并表明这种方法对高危早期乳腺癌患者具有长期益处。











































 京公网安备 11010802027423号
京公网安备 11010802027423号