当前位置:
X-MOL 学术
›
JAMA Intern. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparison of Priority vs Standard US Food and Drug Administration Premarket Approval Review for High-Risk Medical Devices
JAMA Internal Medicine ( IF 22.5 ) Pub Date : 2020-05-01 , DOI: 10.1001/jamainternmed.2020.0297 Caroline Ong 1 , Vy K Ly 2 , Rita F Redberg 3, 4
JAMA Internal Medicine ( IF 22.5 ) Pub Date : 2020-05-01 , DOI: 10.1001/jamainternmed.2020.0297 Caroline Ong 1 , Vy K Ly 2 , Rita F Redberg 3, 4
Affiliation
|
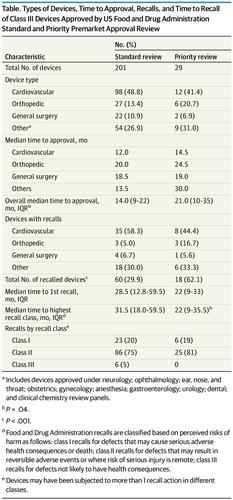
This study compares US Food and Drug Administration premarket approval review times for priority vs standard review for high-risk devices.
中文翻译:

优先与标准美国食品和药物管理局对高风险医疗器械的上市前批准审查的比较
本研究比较了美国食品和药物管理局上市前批准的优先审查时间与高风险设备的标准审查时间。
更新日期:2020-05-01
中文翻译:

优先与标准美国食品和药物管理局对高风险医疗器械的上市前批准审查的比较
本研究比较了美国食品和药物管理局上市前批准的优先审查时间与高风险设备的标准审查时间。











































 京公网安备 11010802027423号
京公网安备 11010802027423号