当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper-catalyzed remote C–H arylation of polycyclic aromatic hydrocarbons (PAHs)
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-03-30 , DOI: 10.3762/bjoc.16.49 Anping Luo , Min Zhang , Zhangyi Fu , Jingbo Lan , Di Wu , Jingsong You
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-03-30 , DOI: 10.3762/bjoc.16.49 Anping Luo , Min Zhang , Zhangyi Fu , Jingbo Lan , Di Wu , Jingsong You
|
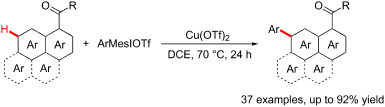
The regioselective C–H arylation of substituted polycyclic aromatic hydrocarbons (PAHs) is a desired but challenging task. A copper-catalyzed C7–H arylation of 1-naphthamides has been developed by using aryliodonium salts as arylating reagents. This protocol does not need to use precious metal catalysts and tolerates wide variety of functional groups. Under standard conditions, the remote C–H arylation of other PAHs including phenanthrene-9-carboxamide, pyrene-1-carboxamide and fluoranthene-3-carboxamide has also accomplished, which provides an opportunity for the development of diverse organic optoelectronic materials.
中文翻译:

铜催化的多环芳烃(PAHs)的远程C–H芳基化
取代的多环芳烃(PAHs)的区域选择性C–H芳基化是一项理想而艰巨的任务。通过使用芳基碘鎓盐作为芳基化试剂,开发了铜催化的1-萘酰胺的C7–H芳基化反应。该协议不需要使用贵金属催化剂,并且可以耐受多种官能团。在标准条件下,还可以完成其他PAH的远程C–H芳基化反应,包括菲-9甲酰胺,pyr 1甲酰胺和荧蒽3甲酰胺,这为开发多种有机光电材料提供了机会。
更新日期:2020-03-30
中文翻译:

铜催化的多环芳烃(PAHs)的远程C–H芳基化
取代的多环芳烃(PAHs)的区域选择性C–H芳基化是一项理想而艰巨的任务。通过使用芳基碘鎓盐作为芳基化试剂,开发了铜催化的1-萘酰胺的C7–H芳基化反应。该协议不需要使用贵金属催化剂,并且可以耐受多种官能团。在标准条件下,还可以完成其他PAH的远程C–H芳基化反应,包括菲-9甲酰胺,pyr 1甲酰胺和荧蒽3甲酰胺,这为开发多种有机光电材料提供了机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号