当前位置:
X-MOL 学术
›
Cytotherapy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Expansion and CD2/CD3/CD28 stimulation enhance Th2 cytokine secretion of human invariant NKT cells with retained anti-tumor cytotoxicity
Cytotherapy ( IF 3.7 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.jcyt.2020.01.011 Kelly Andrews 1 , Anouk A J Hamers 2 , Xiaodian Sun 3 , Geoffrey Neale 4 , Katherine Verbist 5 , Paige Tedrick 5 , Kim E Nichols 5 , Shalini Pereira 6 , Daniel E Geraghty 7 , Asha B Pillai 1
Cytotherapy ( IF 3.7 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.jcyt.2020.01.011 Kelly Andrews 1 , Anouk A J Hamers 2 , Xiaodian Sun 3 , Geoffrey Neale 4 , Katherine Verbist 5 , Paige Tedrick 5 , Kim E Nichols 5 , Shalini Pereira 6 , Daniel E Geraghty 7 , Asha B Pillai 1
Affiliation
|
BACKGROUND AIMS
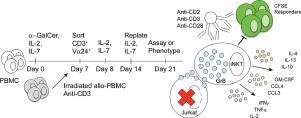
Key obstacles in human iNKT cell translational research and immunotherapy include the lack of robust protocols for dependable expansion of human iNKT cells and the paucity of data on phenotypes in post-expanded cells. METHODS
We delineate expansion methods using interleukin (IL)-2, IL-7 and allogeneic feeder cells and anti-CD2/CD3/CD28 stimulation by which to dependably augment Th2 polarization and direct cytotoxicity of human peripheral blood CD3+Vα24+Vβ11+ iNKT cells. RESULTS
Gene and protein expression profiling demonstrated augmented Th2 cytokine secretion (IL-4, IL-5, IL-13) in expanded iNKT cells stimulated with anti-CD2/CD3/CD28 antibodies. Cytotoxic effector molecules including granzyme B were increased in expanded iNKT cells after CD2/CD3/CD28 stimulation. Direct cytotoxicity assays using unstimulated expanded iNKT cell effectors revealed α-galactosyl ceramide (α-GalCer)-dependent killing of the T-ALL cell line Jurkat. Moreover, CD2/CD3/CD28 stimulation of expanded iNKT cells augmented their (α-GalCer-independent) killing of Jurkat cells. Co-culture of expanded iNKT cells with stimulated responder cells confirmed contact-dependent inhibition of activated CD4+ and CD8+ responder T cells. DISCUSSION
These data establish a robust protocol to expand and novel pathways to enhance Th2 cytokine secretion and direct cytotoxicity in human iNKT cells, findings with direct implications for autoimmunity, vaccine augmentation and anti-infective immunity, cancer immunotherapy and transplantation.
中文翻译:

扩增和 CD2/CD3/CD28 刺激增强人不变 NKT 细胞的 Th2 细胞因子分泌,并保留抗肿瘤细胞毒性
背景目标人类 iNKT 细胞转化研究和免疫治疗的主要障碍包括缺乏可靠的人类 iNKT 细胞扩增方案,以及扩增后细胞表型数据的缺乏。方法我们描述了使用白细胞介素 (IL)-2、IL-7 和同种异体饲养细胞和抗 CD2/CD3/CD28 刺激的扩增方法,通过这些方法可靠地增强人外周血 CD3+Vα24+Vβ11+ iNKT 细胞的 Th2 极化和直接细胞毒性. 结果 基因和蛋白质表达谱表明,在用抗 CD2/CD3/CD28 抗体刺激的扩增 iNKT 细胞中,Th2 细胞因子分泌增加(IL-4、IL-5、IL-13)。CD2/CD3/CD28 刺激后,扩增的 iNKT 细胞中包括颗粒酶 B 在内的细胞毒性效应分子增加。使用未刺激的扩增 iNKT 细胞效应器进行的直接细胞毒性测定显示,α-半乳糖神经酰胺 (α-GalCer) 依赖于 T-ALL 细胞系 Jurkat 的杀伤。此外,CD2/CD3/CD28 对扩增的 iNKT 细胞的刺激增强了它们对 Jurkat 细胞的(α-GalCer 依赖性)杀伤。扩增的 iNKT 细胞与刺激的应答细胞的共培养证实了对活化的 CD4+ 和 CD8+ 应答 T 细胞的接触依赖性抑制。讨论这些数据建立了一个强大的协议来扩展和新途径来增强人类 iNKT 细胞中 Th2 细胞因子的分泌和直接细胞毒性,这些发现对自身免疫、疫苗增强和抗感染免疫、癌症免疫治疗和移植有直接影响。扩增的 iNKT 细胞的 CD2/CD3/CD28 刺激增强了它们(α-GalCer 非依赖性)对 Jurkat 细胞的杀伤。扩增的 iNKT 细胞与刺激的应答细胞的共培养证实了对活化的 CD4+ 和 CD8+ 应答 T 细胞的接触依赖性抑制。讨论这些数据建立了一个强大的协议来扩展和新途径来增强人类 iNKT 细胞中 Th2 细胞因子的分泌和直接细胞毒性,这些发现对自身免疫、疫苗增强和抗感染免疫、癌症免疫治疗和移植有直接影响。扩增的 iNKT 细胞的 CD2/CD3/CD28 刺激增强了它们(α-GalCer 非依赖性)对 Jurkat 细胞的杀伤。扩增的 iNKT 细胞与刺激的应答细胞的共培养证实了对活化的 CD4+ 和 CD8+ 应答 T 细胞的接触依赖性抑制。讨论这些数据建立了一个强大的协议来扩展和新途径来增强人类 iNKT 细胞中 Th2 细胞因子的分泌和直接细胞毒性,这些发现对自身免疫、疫苗增强和抗感染免疫、癌症免疫治疗和移植有直接影响。
更新日期:2020-05-01
中文翻译:

扩增和 CD2/CD3/CD28 刺激增强人不变 NKT 细胞的 Th2 细胞因子分泌,并保留抗肿瘤细胞毒性
背景目标人类 iNKT 细胞转化研究和免疫治疗的主要障碍包括缺乏可靠的人类 iNKT 细胞扩增方案,以及扩增后细胞表型数据的缺乏。方法我们描述了使用白细胞介素 (IL)-2、IL-7 和同种异体饲养细胞和抗 CD2/CD3/CD28 刺激的扩增方法,通过这些方法可靠地增强人外周血 CD3+Vα24+Vβ11+ iNKT 细胞的 Th2 极化和直接细胞毒性. 结果 基因和蛋白质表达谱表明,在用抗 CD2/CD3/CD28 抗体刺激的扩增 iNKT 细胞中,Th2 细胞因子分泌增加(IL-4、IL-5、IL-13)。CD2/CD3/CD28 刺激后,扩增的 iNKT 细胞中包括颗粒酶 B 在内的细胞毒性效应分子增加。使用未刺激的扩增 iNKT 细胞效应器进行的直接细胞毒性测定显示,α-半乳糖神经酰胺 (α-GalCer) 依赖于 T-ALL 细胞系 Jurkat 的杀伤。此外,CD2/CD3/CD28 对扩增的 iNKT 细胞的刺激增强了它们对 Jurkat 细胞的(α-GalCer 依赖性)杀伤。扩增的 iNKT 细胞与刺激的应答细胞的共培养证实了对活化的 CD4+ 和 CD8+ 应答 T 细胞的接触依赖性抑制。讨论这些数据建立了一个强大的协议来扩展和新途径来增强人类 iNKT 细胞中 Th2 细胞因子的分泌和直接细胞毒性,这些发现对自身免疫、疫苗增强和抗感染免疫、癌症免疫治疗和移植有直接影响。扩增的 iNKT 细胞的 CD2/CD3/CD28 刺激增强了它们(α-GalCer 非依赖性)对 Jurkat 细胞的杀伤。扩增的 iNKT 细胞与刺激的应答细胞的共培养证实了对活化的 CD4+ 和 CD8+ 应答 T 细胞的接触依赖性抑制。讨论这些数据建立了一个强大的协议来扩展和新途径来增强人类 iNKT 细胞中 Th2 细胞因子的分泌和直接细胞毒性,这些发现对自身免疫、疫苗增强和抗感染免疫、癌症免疫治疗和移植有直接影响。扩增的 iNKT 细胞的 CD2/CD3/CD28 刺激增强了它们(α-GalCer 非依赖性)对 Jurkat 细胞的杀伤。扩增的 iNKT 细胞与刺激的应答细胞的共培养证实了对活化的 CD4+ 和 CD8+ 应答 T 细胞的接触依赖性抑制。讨论这些数据建立了一个强大的协议来扩展和新途径来增强人类 iNKT 细胞中 Th2 细胞因子的分泌和直接细胞毒性,这些发现对自身免疫、疫苗增强和抗感染免疫、癌症免疫治疗和移植有直接影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号