Chemical Physics ( IF 2.0 ) Pub Date : 2020-03-30 , DOI: 10.1016/j.chemphys.2020.110777 Mitradip Das , Sandeep Dash , B.L. Bhargava
|
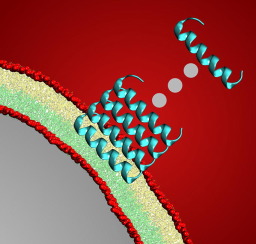
Phenol soluble modulin (PSM) 3, the most toxic member of -toxin in Staphylococcus aureus bacteria, forms cross- amyloid fibrils and is selectively toxic to the mammalian cell membranes. In this work, it has been discovered that hydrophobic interactions play a major role in fibril formation of PSM-3 strands, with stabilization energy of 28.7 kCal mol-1. We considered two model bilayers mimicking mammalian and bacterial cell membranes, and found that single -helix strand penetration is energetically unfavorable in both of them. Hence, we propose a simple model using energetics to understand the reason for selective toxicity of the peptide to the mammalian cell membrane. This study, besides enhancing the understanding of PSM-3, can also act as a stepping stone in future drug development against S. aureus.
中文翻译:

颤动引起交叉选择性细胞毒性的计算研究 淀粉样蛋白–苯酚可溶性莫德林 3
苯酚可溶调节蛋白(PSM) 3,最毒的成员 -金黄色葡萄球菌细菌中的毒素形成交叉-淀粉样原纤维,对哺乳动物细胞膜有选择性的毒性。在这项工作中,我们发现疏水相互作用在PSM-的原纤维形成中起主要作用3股,稳定能量为28.7 kCal mol -1。我们考虑了两个模仿哺乳动物和细菌细胞膜的双层模型,发现-螺旋线在两者中都在能量上不利。因此,我们提出了一个使用能量学的简单模型,以了解该肽对哺乳动物细胞膜选择性毒性的原因。这项研究除了增进对PSM的理解外,3,在将来针对金黄色葡萄球菌的药物开发中也可以充当垫脚石。









































 京公网安备 11010802027423号
京公网安备 11010802027423号