Chemical Physics Letters ( IF 2.8 ) Pub Date : 2020-03-29 , DOI: 10.1016/j.cplett.2020.137415 Dan Zhao , Yang Liu , Wenjie Fan
|
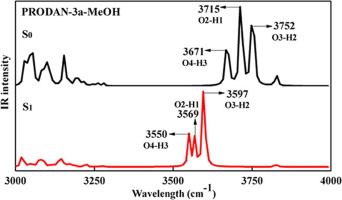
The role of intermolecular hydrogen bond of PRODAN derivative is investigated based on density functional theory (DFT) and time-dependent density functional theory (TDDFT). Geometry analysis demonstrated that the intermolecular hydrogen bonds are enhanced in S1 state. The red-shift phenomenon of fluorescence spectrum and IR spectrum as well as the energy of intermolecular hydrogen bond further confirm the intermolecular hydrogen bond strengthening in excited state. PRODAN-3a-MeOH has been electronically excited to S1 which is a local excitation. The small change of electron density occurred the interaction region of intermolecular can directly influence the intermolecular hydrogen bonding in S1 state.
中文翻译:

分子间氢键在一种PRODAN衍生物与甲醇的激发态微溶剂化中的作用
基于密度泛函理论(DFT)和时间相关的密度泛函理论(TDDFT)研究了PRODAN衍生物的分子间氢键的作用。几何分析表明,在S 1状态下分子间氢键得到增强。荧光光谱和IR光谱的红移现象以及分子间氢键的能量进一步证实了在激发态下分子间氢键的增强。PRODAN-3a-MeOH已被电子激发至S 1,这是局部激发。电子密度的微小变化发生在分子间的相互作用区域可以直接影响S 1状态下的分子间氢键。



























 京公网安备 11010802027423号
京公网安备 11010802027423号