当前位置:
X-MOL 学术
›
J. Colloid Interface Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revisiting thioflavin T (ThT) fluorescence as a marker of protein fibrillation - The prominent role of electrostatic interactions.
Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2020-03-30 , DOI: 10.1016/j.jcis.2020.03.075 Elad Arad 1 , Hodaya Green 2 , Raz Jelinek 1 , Hanna Rapaport 3
Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2020-03-30 , DOI: 10.1016/j.jcis.2020.03.075 Elad Arad 1 , Hodaya Green 2 , Raz Jelinek 1 , Hanna Rapaport 3
Affiliation
|
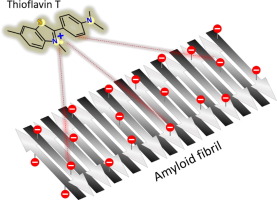
Thioflavin T (ThT), a benzothiazole-based fluorophore, is a prominent dye widely employed for monitoring amyloid fibril assembly. Despite the near-universal presumption that ThT binds to β-sheet domains upon fibrillar surface via hydrophobic forces, the contribution of the positive charge of ThT to fibril binding and concomitant fluorescence enhancement have not been thoroughly assessed. Here we demonstrate a considerable interdependence between ThT fluorescence and electrostatic charges of peptide fibrils. Specifically, by analyzing both fibril-forming synthetic peptides and prominent natural fibrillar peptides, we demonstrate pronounced modulations of ThT fluorescence signal that were solely dependent upon electrostatic interactions between ThT and peptide surface. The results further attest to the fact that fibril ζ-potential rather than pH-dependent assembly of the fibrils constitute the primary factor affecting ThT binding and fluorescence. This study provides the first quantitative assessment of electrostatically driven ThT fluorescence upon adsorption to amyloid fibrils.
中文翻译:

回顾硫代黄素T(ThT)荧光作为蛋白原纤化的标志-静电相互作用的突出作用。
硫黄素T(ThT),一种基于苯并噻唑的荧光团,是一种广泛用于监测淀粉样蛋白原纤维组装的杰出染料。尽管几乎普遍地认为ThT通过疏水力与原纤维表面的β-折叠结构域结合,但是尚未完全评估ThT的正电荷对原纤维结合和伴随的荧光增强的贡献。在这里,我们证明了ThT荧光与肽原纤维的静电荷之间有很大的相互依赖性。具体而言,通过分析形成原纤维的合成肽和突出的天然原纤维肽,我们证明了ThT荧光信号的明显调节,其仅取决于ThT与肽表面之间的静电相互作用。该结果进一步证明了以下事实:原纤维的ζ电势而不是pH依赖性的原纤维构成影响ThT结合和荧光的主要因素。这项研究提供了对淀粉样原纤维吸附后静电驱动的ThT荧光的首次定量评估。
更新日期:2020-03-31
中文翻译:

回顾硫代黄素T(ThT)荧光作为蛋白原纤化的标志-静电相互作用的突出作用。
硫黄素T(ThT),一种基于苯并噻唑的荧光团,是一种广泛用于监测淀粉样蛋白原纤维组装的杰出染料。尽管几乎普遍地认为ThT通过疏水力与原纤维表面的β-折叠结构域结合,但是尚未完全评估ThT的正电荷对原纤维结合和伴随的荧光增强的贡献。在这里,我们证明了ThT荧光与肽原纤维的静电荷之间有很大的相互依赖性。具体而言,通过分析形成原纤维的合成肽和突出的天然原纤维肽,我们证明了ThT荧光信号的明显调节,其仅取决于ThT与肽表面之间的静电相互作用。该结果进一步证明了以下事实:原纤维的ζ电势而不是pH依赖性的原纤维构成影响ThT结合和荧光的主要因素。这项研究提供了对淀粉样原纤维吸附后静电驱动的ThT荧光的首次定量评估。











































 京公网安备 11010802027423号
京公网安备 11010802027423号