当前位置:
X-MOL 学术
›
Comp. Mater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ab initio thermodynamics studies on the phase stability of PtO2 under ambient and high-pressure conditions
Computational Materials Science ( IF 3.1 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.commatsci.2020.109708 Quan Chen , Yong Yang
Computational Materials Science ( IF 3.1 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.commatsci.2020.109708 Quan Chen , Yong Yang
|
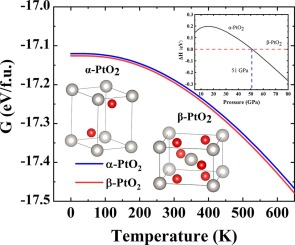
Abstract We studied the structural and thermodynamic properties of platinum dioxide (PtO2) at ambient and high pressures by using ab initio thermodynamics calculations. The Gibbs free energies of two stable phases of PtO2, α-PtO2 and β-PtO2, were calculated, and the latter turns out to be energetically the most stable within a wide range of temperature (0–600 K) and pressure (0-51GPa). To study the thermodynamic stability of PtO2, we have developed a method to effectively calculate the entropy and Gibbs free energies of O2 molecules at gas phase. Using the free energy data of O2, that of PtO2 and bulk Pt which are calculated with the quasi-harmonic approximation (QHA) and finite temperature correction, we showed that β-PtO2 will decompose into Pt and O2 at around 646 K, comparing well with experimental observation. Based on first-principles calculations and particle-swarm optimization (PSO) method for structure search, we have investigated the high-pressure behavior of PtO2. It is predicted that β-PtO2 will be transformed to α-PtO2 at a pressure of ~51 GPa. In addition, we found that temperature plays a minor role in the β-to-α phase transformation.
中文翻译:

PtO2 在常压和高压条件下相稳定性的从头算热力学研究
摘要 我们通过从头算热力学计算研究了二氧化铂 (PtO2) 在常压和高压下的结构和热力学性质。计算了 PtO2 两个稳定相(α-PtO2 和 β-PtO2)的吉布斯自由能,结果证明后者在很宽的温度(0-600 K)和压力(0- 51GPa)。为了研究 PtO2 的热力学稳定性,我们开发了一种有效计算气相 O2 分子的熵和吉布斯自由能的方法。使用通过准谐波近似(QHA)和有限温度校正计算的 O2、PtO2 和体 Pt 的自由能数据,我们表明 β-PtO2 将在 646 K 左右分解为 Pt 和 O2,比较良好与实验观察。基于第一性原理计算和用于结构搜索的粒子群优化 (PSO) 方法,我们研究了 PtO2 的高压行为。预计β-PtO2 将在~51 GPa 的压力下转化为α-PtO2。此外,我们发现温度在 β 到 α 相变中起次要作用。
更新日期:2020-07-01
中文翻译:

PtO2 在常压和高压条件下相稳定性的从头算热力学研究
摘要 我们通过从头算热力学计算研究了二氧化铂 (PtO2) 在常压和高压下的结构和热力学性质。计算了 PtO2 两个稳定相(α-PtO2 和 β-PtO2)的吉布斯自由能,结果证明后者在很宽的温度(0-600 K)和压力(0- 51GPa)。为了研究 PtO2 的热力学稳定性,我们开发了一种有效计算气相 O2 分子的熵和吉布斯自由能的方法。使用通过准谐波近似(QHA)和有限温度校正计算的 O2、PtO2 和体 Pt 的自由能数据,我们表明 β-PtO2 将在 646 K 左右分解为 Pt 和 O2,比较良好与实验观察。基于第一性原理计算和用于结构搜索的粒子群优化 (PSO) 方法,我们研究了 PtO2 的高压行为。预计β-PtO2 将在~51 GPa 的压力下转化为α-PtO2。此外,我们发现温度在 β 到 α 相变中起次要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号