当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of thienopyridinones via hydrazide-alkyne cyclization
Tetrahedron ( IF 2.1 ) Pub Date : 2020-03-30 , DOI: 10.1016/j.tet.2020.131151 Nalan Korkmaz Cokol , Kübra Erden , Furkan Melih Gunay , Cagatay Dengiz , Metin Balci
中文翻译:

thienopyridinones的合成通过酰肼炔环化
更新日期:2020-03-30
Tetrahedron ( IF 2.1 ) Pub Date : 2020-03-30 , DOI: 10.1016/j.tet.2020.131151 Nalan Korkmaz Cokol , Kübra Erden , Furkan Melih Gunay , Cagatay Dengiz , Metin Balci
|
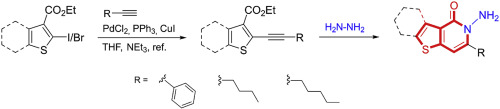
A novel and efficient method for the synthesis of thieno[3,2-c]pyridinones have been developed. The synthetic strategy relies on the synthesis of halothiophene esters utilized as substrates for subsequent Sonogashira cross-coupling reactions. Hydrazinolysis of corresponding esters and following hydrazide-alkyne ring closure transformations resulted in target thienopyridinones in good yields. It is also shown that pure hydrazine monohydrate is required to facilitate the hydrazide formations.
中文翻译:

thienopyridinones的合成通过酰肼炔环化
已经开发了新颖且有效的合成噻吩并[3,2-c]吡啶酮的方法。合成策略依赖于卤代噻吩酯的合成,该卤代噻吩酯用作后续Sonogashira交叉偶联反应的底物。相应酯的水合肼解反应以及酰肼-炔烃的闭环转化产生了高产率的目标噻吩并吡啶酮。还表明需要纯一水合肼来促进酰肼的形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号