当前位置:
X-MOL 学术
›
J. Chem. Educ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Relating ΔHvap of Organic Liquids to Intermolecular Forces: Simple Modifications of a Classic General Chemistry Experiment
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2020-03-30 , DOI: 10.1021/acs.jchemed.0c00163 Jeffrey P. Fitzgerald 1 , Robert F. Ferrante 1 , Michael Brown 1 , Jonathan Cabarrus 1
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2020-03-30 , DOI: 10.1021/acs.jchemed.0c00163 Jeffrey P. Fitzgerald 1 , Robert F. Ferrante 1 , Michael Brown 1 , Jonathan Cabarrus 1
Affiliation
|
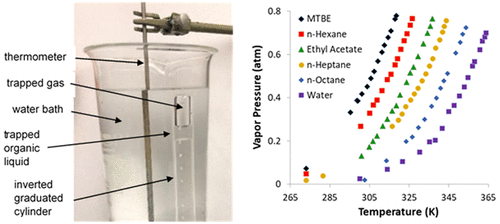
The concept of equilibrium vapor pressure plays a key role in the general chemistry curriculum; it is among the first and most easily demonstrated examples of equilibrium and frequently caps off the first semester of general chemistry where it illustrates the properties of liquids and intermolecular forces. We report here simple modifications of Levinson’s classic experiment that allow measurement of vapor pressures for organic samples at a variety of temperatures. Data analysis gives heats of vaporization which may be interpreted in terms of intermolecular attractive forces. Equipment needs are simple and readily available: a 10 mL graduated cylinder, a tall 1 L beaker, disposable graduated syringes, and a 10 in. needle or cannula bent into a “J” shape. The method uses 1 mL or less of organic sample per trial and works well for organic liquids that are immiscible with and are less dense than water. Student results are presented for the experiment conducted over three semesters as well as the impact on student learning as assessed by common exam results and end-of-semester student surveys.
中文翻译:

将有机液体的ΔH蒸气与分子间力相关:经典常规化学实验的简单修改
平衡蒸气压的概念在普通化学课程中起着关键作用。它是第一个也是最容易证明的平衡实例,并且经常在一般化学的第一个学期结束时用它来说明液体的性质和分子间力。我们在这里报告了Levinson经典实验的简单修改,该实验允许在各种温度下测量有机样品的蒸气压。数据分析给出了汽化热,可以根据分子间吸引力来解释。设备需求简单易用:一个10毫升量筒,一个1 L高烧杯,一次性量筒和一个弯曲成“ J”形的10英寸针头或套管。该方法每次试验使用1 mL或更少的有机样品,对于与水不混溶且密度小于水的有机液体效果很好。展示了三个学期进行的实验的学生成绩,以及通过普通考试成绩和学期末学生调查评估的对学生学习的影响。
更新日期:2020-03-30
中文翻译:

将有机液体的ΔH蒸气与分子间力相关:经典常规化学实验的简单修改
平衡蒸气压的概念在普通化学课程中起着关键作用。它是第一个也是最容易证明的平衡实例,并且经常在一般化学的第一个学期结束时用它来说明液体的性质和分子间力。我们在这里报告了Levinson经典实验的简单修改,该实验允许在各种温度下测量有机样品的蒸气压。数据分析给出了汽化热,可以根据分子间吸引力来解释。设备需求简单易用:一个10毫升量筒,一个1 L高烧杯,一次性量筒和一个弯曲成“ J”形的10英寸针头或套管。该方法每次试验使用1 mL或更少的有机样品,对于与水不混溶且密度小于水的有机液体效果很好。展示了三个学期进行的实验的学生成绩,以及通过普通考试成绩和学期末学生调查评估的对学生学习的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号