当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Equilibrium Solubility Determination and Correlation of Monobenzone in Fifteen Monosolvents at a Series of Temperatures
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-03-30 , DOI: 10.1021/acs.jced.9b00817 Rongrong Li 1 , Wei Wang 1 , Xianlang Chen 1 , Hong Chen 1 , Huanhuan Bao 1 , Yuwei Zhu 1 , Jia Zhao 2 , Deman Han 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-03-30 , DOI: 10.1021/acs.jced.9b00817 Rongrong Li 1 , Wei Wang 1 , Xianlang Chen 1 , Hong Chen 1 , Huanhuan Bao 1 , Yuwei Zhu 1 , Jia Zhao 2 , Deman Han 1
Affiliation
|
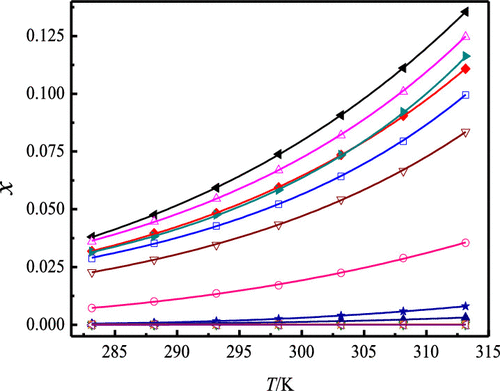
This study reports the equilibrium solubility for monobenzone in methanol, ethanol, n-propanol, isopropanol, n-butanol, acetonitrile, ethyl acetate, acetone, 1,4-dioxane, cyclohexane, N,N-dimethylformamide (DMF), N-methyl-2-pyrrolidinone (NMP), n-hexane, n-octanol, and water that was determined by means of the isothermal method between 283.15 and 313.15 K under p = 101.1 kPa. The maximum value of monobenzone solubility was 0.6316 in mole fraction at 313.15 K dissolved in ethyl acetate, but the least data was achieved in water. The solubility of monobenzone increased with the rising temperature and the order followed in the 15 pure solvents was ethyl acetate (0.6316, 313.15 K) > 1,4-dioxane (0.4350, 313.15 K) > methanol (0.1356, 313.15 K) > acetonitrile (0.1247, 313.15 K) > (ethanol, isopropanol) > n-propanol (0.09942, 313.15 K) > n-butanol (0.08346, 313.15 K) > n-octanol (0.03546, 313.15 K) > cyclohexane (8.020×10–3, 313.15 K) > n-hexane (3.133 × 10–3, 313.15 K) > NMP (2.372 × 10–4, 313.15 K) > DMF (1.145 × 10–4, 313.15 K) > acetone (4.032 × 10–5, 313.15 K) > water (3.019 × 10–5, 313.15 K). There was no existence of processes such as solvation or polymorphic transformation during the entire experiment. Several models covering the modified Apelblat, λh, Wilson, and non-random two-liquid models were applied to correlate and calculate monobenzone solubility data in fifteen monosolvents. Results showed that the modified Apelblat equation provided the best results with the experimental ones. The largest relative average deviation and root-mean-square deviation were no more than 4.74 × 10–2 and 1.81 × 10–3, respectively.
中文翻译:

一系列温度下十五种单溶剂中一苯并二甲苯的平衡溶解度测定和相关性
这项研究报告了单苯甲醚在甲醇,乙醇,正丙醇,异丙醇,正丁醇,乙腈,乙酸乙酯,丙酮,1,4-二恶烷,环己烷,N,N-二甲基甲酰胺(DMF),N-甲基中的平衡溶解度-2-吡咯烷酮(NMP),ñ己烷,ñ,是由等温方法的手段和283.15 313.15 K的下测定辛醇和水p= 101.1 kPa。在313.15 K下,溶解在乙酸乙酯中的二苯甲酮溶解度的最大值(摩尔分数)为0.6316,但在水中获得的数据最少。单苯甲酮的溶解度随温度的升高而增加,在15种纯溶剂中遵循的顺序为乙酸乙酯(0.6316,313.15 K)> 1,4-二恶烷(0.4350,313.15 K)>甲醇(0.1356,313.15 K)>乙腈( 0.1247,313.15 K)>(乙醇,异丙醇)>正丙醇(0.09942,313.15 K)>正丁醇(0.08346,313.15 K)>正辛醇(0.03546,313.15 K)>环己烷(8.020×10 –3) 313.15 K)> ñ -己烷(3.133×10 -3,313.15 K)> NMP(2.372×10 -4,313.15 K)> DMF(1。-4,313.15 K)>丙酮(4.032×10 -5,313.15 K)>水(3.019×10 -5,313.15 K)。在整个实验过程中不存在诸如溶剂化或多晶型转化的过程。几种模式覆盖改性Apelblat,λ ħ,威尔逊,和非随机双液体模型15个monosolvents分别适用于关联和计算莫诺苯宗溶解度数据。结果表明,改进后的Apelblat方程与实验结果吻合最好。最大相对平均偏差和均方根偏差分别不超过4.74×10 –2和1.81×10 –3。
更新日期:2020-03-30
中文翻译:

一系列温度下十五种单溶剂中一苯并二甲苯的平衡溶解度测定和相关性
这项研究报告了单苯甲醚在甲醇,乙醇,正丙醇,异丙醇,正丁醇,乙腈,乙酸乙酯,丙酮,1,4-二恶烷,环己烷,N,N-二甲基甲酰胺(DMF),N-甲基中的平衡溶解度-2-吡咯烷酮(NMP),ñ己烷,ñ,是由等温方法的手段和283.15 313.15 K的下测定辛醇和水p= 101.1 kPa。在313.15 K下,溶解在乙酸乙酯中的二苯甲酮溶解度的最大值(摩尔分数)为0.6316,但在水中获得的数据最少。单苯甲酮的溶解度随温度的升高而增加,在15种纯溶剂中遵循的顺序为乙酸乙酯(0.6316,313.15 K)> 1,4-二恶烷(0.4350,313.15 K)>甲醇(0.1356,313.15 K)>乙腈( 0.1247,313.15 K)>(乙醇,异丙醇)>正丙醇(0.09942,313.15 K)>正丁醇(0.08346,313.15 K)>正辛醇(0.03546,313.15 K)>环己烷(8.020×10 –3) 313.15 K)> ñ -己烷(3.133×10 -3,313.15 K)> NMP(2.372×10 -4,313.15 K)> DMF(1。-4,313.15 K)>丙酮(4.032×10 -5,313.15 K)>水(3.019×10 -5,313.15 K)。在整个实验过程中不存在诸如溶剂化或多晶型转化的过程。几种模式覆盖改性Apelblat,λ ħ,威尔逊,和非随机双液体模型15个monosolvents分别适用于关联和计算莫诺苯宗溶解度数据。结果表明,改进后的Apelblat方程与实验结果吻合最好。最大相对平均偏差和均方根偏差分别不超过4.74×10 –2和1.81×10 –3。











































 京公网安备 11010802027423号
京公网安备 11010802027423号